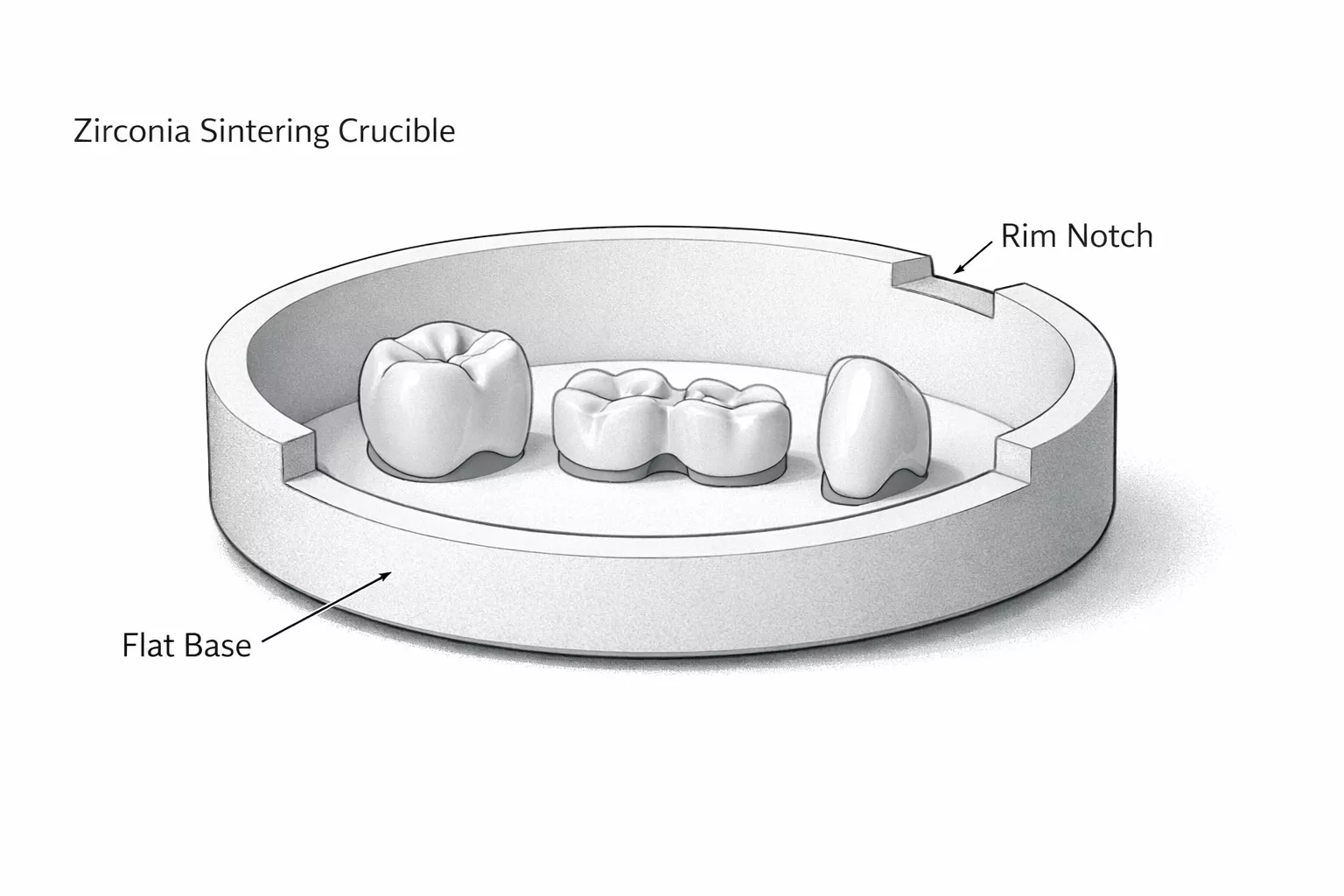
Laboratory Alumina Crucible performance determines the reliability, purity, and repeatability of advanced laboratory workflows.
This guide offers a detailed technical overview of Laboratory Alumina Crucibles, describing their material foundations, structural advantages, performance characteristics, and essential laboratory applications. Each section provides quantifiable parameters and engineering context to support precise selection and operation.
What Are Laboratory Alumina Crucibles?
Laboratory Alumina Crucible usage begins with understanding the material fundamentals that define its performance in thermal, chemical, and analytical environments.
Definition and Material Composition
Laboratory Alumina Crucibles are high-temperature containment vessels manufactured primarily from sintered alumina (Al₂O₃), typically achieving purity levels between 95% and 99.8%. Because alumina exhibits a melting point of approximately 2050 °C, the material provides a reliable platform for thermal processing. Additionally, its crystalline α-phase structured α-Al₂O₃1 ensures dimensional stability during repeated heating cycles.
Moreover, Laboratory Alumina Crucibles offer consistent thermal behavior because their sintered microstructure achieves densities above 3.80 g/cm³, significantly reducing permeability. These density levels enhance both temperature resistance and chemical inertness while supporting application stability in laboratories. Furthermore, controlled grain size between 2–8 µm improves mechanical strength, allowing the crucibles to endure various thermal gradients.
Consequently, the engineered purity and crystalline phase of these crucibles position them as essential components in workflows requiring clean, repeatable, and contamination-free experimental outcomes.
| Material Composition (wt%) | Typical Range |
|---|---|
| Al₂O₃ Purity (%) | 95–99.8 |
| SiO₂ Impurity (%) | 0.02–0.20 |
| MgO Additives (%) | 0.01–0.10 |
| Fe₂O₃ Impurity (%) | <0.03 |
Key Differences from General Ceramic Crucibles
Laboratory Alumina Crucibles differ from general ceramic crucibles through their superior purity, higher density, greater thermal capacity, and significantly enhanced chemical stability. Since general ceramic crucibles often contain mixed oxides and clay-based materials, their performance typically degrades above 1200 °C, whereas alumina maintains integrity up to 1700–1800 °C.
Additionally, Laboratory Alumina Crucibles exhibit porosity levels below 1%, while conventional ceramic crucibles may exceed 5%, increasing risks of contamination, localized reactions, and absorption of reactive species. This distinction becomes critical in analytical workflows requiring consistent background values. Furthermore, alumina provides compressive strengths exceeding 250 MPa, allowing it to withstand handling stress and rapid thermal cycling.
Therefore, the performance gap between general ceramics and Laboratory Alumina Crucibles directly influences laboratory reliability, particularly in processes involving [calcination])(https://www.sciencedirect.com/topics/materials-science/calcination)[^2], ashing2, or high-purity material preparation.
| Property | Laboratory Alumina Crucible | General Ceramic Crucible |
|---|---|---|
| Maximum Working Temperature (°C) | 1700–1800 | 1000–1200 |
| Open Porosity (%) | <1 | 5–12 |
| Density (g/cm³) | 3.80–3.95 | 2.20–2.60 |
| Chemical Inertness | High | Moderate |
| Thermal Shock Resistance | Moderate–High | Low–Moderate |

Why Are Laboratory Alumina Crucibles Essential in Laboratories?
Laboratory Alumina Crucible performance is valued because their structural, chemical, and thermal attributes directly enhance laboratory accuracy, workflow safety, and experimental repeatability.
High-Temperature Structural Stability
Laboratory Alumina Crucibles maintain structural integrity at temperatures between 1500 °C and 1700 °C, enabling laboratories to conduct high-temperature reactions without vessel deformation. Because alumina exhibits a thermal expansion coefficient of approximately 8.0 × 10⁻⁶/K, dimensional changes remain predictable and controlled. Therefore, these crucibles support demanding calcination and ashing workflows.
Furthermore, the sintered alumina microstructure produces compressive strengths exceeding 250 MPa, providing resistance to thermal fatigue during repeated cycles. Even under temperature gradients of 50–80 °C/min, alumina maintains internal cohesion due to its stable crystalline α-phase. This stability reduces risks of vessel distortion, ensuring that thermal processes remain precise.
Thus, high-temperature stability positions Laboratory Alumina Crucibles as essential tools for thermal treatment experiments requiring consistency across multiple cycles.
| Temperature Performance Metric | Laboratory Alumina Crucible |
|---|---|
| Maximum Working Temperature (°C) | 1700–1800 |
| Recommended Ramp Rate (°C/min) | 5–10 |
| Thermal Expansion Coefficient (×10⁻⁶/K) | 8.0 |
| Compressive Strength (MPa) | 250–300 |
Chemical Resistance to Acids, Bases, and Oxidizing Media
Laboratory Alumina Crucibles offer chemical resistance across diverse laboratory environments because alumina reacts minimally with acidic, basic, or oxidizing media. With impurity levels below 0.1%, contamination risk remains low during high-temperature reactions. Moreover, the dense crystalline lattice hinders the penetration of aggressive chemical species.
Additionally, alumina maintains chemical stability up to 1600 °C in oxidizing conditions and withstands moderate exposure to basic solutions without structural degradation. The material's low open porosity, typically below 1%, further limits internal absorption of reactants. As a result, analytical processes involving catalysts, ceramics, or metallic compounds remain uncontaminated.
Therefore, chemical inertness enhances the reliability and reproducibility of laboratory workflows that depend on clean reaction interfaces.
| Chemical Condition | Stability Level | Supporting Parameter |
|---|---|---|
| Acidic Media | High | Porosity <1% |
| Oxidizing Media | Very High | Reactivity negligible up to 1600 °C |
| Mild Basic Media | Moderate–High | Impurities <0.1% |
| Reducing Atmosphere | Moderate | Reaction risk above 1500 °C |
Resistance to Thermal Shock and Mechanical Stress
Laboratory Alumina Crucibles offer moderate-to-high resistance to thermal shock because their microstructure disperses internal stress across crystalline boundaries. Alumina typically tolerates sudden temperature shifts of 200–300 °C without cracking. Hence, researchers can transition between heating and cooling stages more confidently.
Moreover, mechanical strength plays a central role in maintaining crucible durability. With bending strengths frequently above 150 MPa, alumina withstands accidental impacts or tongs-based handling. The closed microstructure, combined with low porosity, reduces crack propagation during thermal cycling. Consequently, crucibles remain functional under repeated stress.
Thus, resistance to shock and stress ensures that Laboratory Alumina Crucibles remain serviceable throughout extended laboratory use.
| Mechanical Property | Typical Value |
|---|---|
| Bending Strength (MPa) | 150–200 |
| Thermal Shock Tolerance (°C Differential) | 200–300 |
| Fracture Toughness (MPa·m¹ᐟ²) | 3–4 |
| Open Porosity (%) | <1 |
Key Performance Characteristics That Make Laboratory Alumina Crucibles Indispensable
Laboratory Alumina Crucible reliability originates from quantifiable material performance attributes that govern heat transfer, chemical stability, and mechanical strength.
Thermal Conductivity and Heat-Transfer Behavior
Laboratory Alumina Crucibles demonstrate a balanced thermal conductivity range of 18–30 W/m·K, ensuring controlled heat transfer during calcination or high-temperature analysis. Because alumina distributes heat evenly across the crucible wall, temperature gradients remain minimized. Therefore, materials within the crucible experience consistent thermal exposure.
Moreover, the moderate conductivity level prevents overheating at localized points, reducing risks of structural stress. Alumina’s specific heat capacity of approximately 0.9 J/g·K contributes to predictable thermal response during programmed temperature ramps. These properties collectively improve process repeatability, particularly in ceramic powder sintering.
Thus, the thermal behavior of Laboratory Alumina Crucibles improves reaction uniformity and supports precise temperature-driven workflows.
| Thermal Property | Typical Range |
|---|---|
| Thermal Conductivity (W/m·K) | 18–30 |
| Specific Heat Capacity (J/g·K) | 0.85–0.95 |
| Thermal Gradient Uniformity (%) | >90 |
| Recommended Wall Thickness (mm) | 1.0–2.0 |
Microstructure, Porosity, and Material Density
Laboratory Alumina Crucibles are engineered with porosity levels below 1%, significantly limiting absorption of reactive compounds during high-temperature processes. Because high-purity alumina achieves sintered densities of 3.80–3.95 g/cm³, diffusion pathways for contaminants remain restricted. These attributes support clean and reliable sample preparation.
Furthermore, stable α-phase alumina with grain sizes between 2–8 µm ensures reduced crack propagation under thermal stress. Consequently, crucibles maintain dimensional stability even after repeated exposure to high heat. A denser microstructure contributes to improved oxidation resistance across a wide range of laboratory atmospheres.
Therefore, the controlled microstructure of Laboratory Alumina Crucibles serves as the foundation for their chemical, thermal, and mechanical durability.
| Microstructural Metric | Typical Value |
|---|---|
| Density (g/cm³) | 3.80–3.95 |
| Open Porosity (%) | <1 |
| Grain Size (µm) | 2–8 |
| α-Phase Content (%) | >98 |
Mechanical Strength and Wear Resistance
Laboratory Alumina Crucibles exhibit mechanical strengths that safeguard them against fracturing during handling or intensive use. Alumina’s compressive strength often lies between 250–300 MPa, enabling crucibles to withstand clamping, tongs pressure, and stacking. Hence, they remain structurally sound during routine operations.
Additionally, fracture toughness values between 3–4 MPa·m¹ᐟ² protect the crucible from microcrack propagation caused by repetitive thermal cycling. Wear resistance is enhanced by alumina’s hardness, which typically reaches 15–18 GPa on the Vickers scale. Consequently, abrasion from powders or tools does not easily degrade the interior surface.
Thus, mechanical durability strengthens the long-term usability of Laboratory Alumina Crucibles in demanding laboratory conditions.
| Mechanical Metric | Typical Value |
|---|---|
| Compressive Strength (MPa) | 250–300 |
| Fracture Toughness (MPa·m¹ᐟ²) | 3–4 |
| Vickers Hardness (GPa) | 15–18 |
| Wear Rate (mg per cycle) | <0.5 |

Typical Laboratory Applications
Laboratory Alumina Crucible performance demonstrates its value across diverse laboratory workflows where heat stability, purity, and chemical inertness directly determine experimental outcomes.
Laboratory Alumina Crucibles Used for High-Temperature Ashing and Calcination
Laboratory Alumina Crucibles support high-temperature ashing processes because they maintain structural integrity up to 1700 °C. As ashing temperatures typically range from 550–900 °C, alumina offers ample safety margin. Consequently, sample oxidation remains uniform and predictable.
Furthermore, calcination procedures often require heating materials to 900–1200 °C to induce phase transitions or mass-loss reactions. Alumina’s thermal conductivity of 18–30 W/m·K promotes even temperature distribution across the crucible wall. Additionally, the low porosity reduces contamination during mass measurement.
Thus, these crucibles offer a dependable containment environment for thermal decomposition, LOI testing, and phase-transition work.
| Ashing / Calcination Parameter | Typical Value |
|---|---|
| Ashing Temperature (°C) | 550–900 |
| Calcination Temperature (°C) | 900–1200 |
| Temperature Uniformity (%) | >90 |
| Acceptable Thermal Ramp (°C/min) | 5–10 |
Laboratory Alumina Crucibles Applied in Metal Melting and Alloy Work
Laboratory Alumina Crucibles support melting of metals such as aluminum, silver, or copper alloys because their working temperature range exceeds 1500 °C. Since many metal melting processes occur between 660–1100 °C, alumina remains chemically stable throughout. Therefore, it protects metals from undesirable reactions.
Additionally, metals require uniform thermal gradients to avoid oxidation or uneven melting fronts. Alumina’s stable thermal mass, combined with its high compressive strength of 250–300 MPa, allows it to withstand the mechanical stresses of molten metal loading. Moreover, the crucible interior remains resistant to alloy wetting.
Thus, alumina provides a controlled melting environment suitable for metallurgical analysis and small-scale alloy synthesis.
| Metal | Melting Point (°C) | Crucible Stability |
|---|---|---|
| Aluminum | 660 | Excellent |
| Silver | 962 | Excellent |
| Copper Alloy Range | 900–1100 | Excellent |
| Nickel Alloy Range | 1350–1500 | Moderate–High |
Laboratory Alumina Crucibles Support Catalyst Preparation and Solid-State Reactions
Laboratory Alumina Crucibles play a central role in catalyst preparation because reactions often require temperatures above 700 °C and exposure to oxidizing gases. The crucible’s low impurity levels (<0.1%) prevent interference with catalytic surfaces. Consequently, catalyst activation remains consistent.
Additionally, solid-state reactions demand sustained heating at 900–1300 °C, where phase diffusion and lattice transformations occur. Alumina supports these processes due to its high thermal and chemical stability. Furthermore, it endures repeated heat-treatment cycles without structural degradation.
Therefore, alumina vessels ensure clean reaction boundaries essential for catalyst R&D and inorganic synthesis.
| Catalyst Process | Typical Temperature (°C) | Crucible Suitability |
|---|---|---|
| Catalyst Activation | 700–900 | Excellent |
| Calcination Cycles | 800–1100 | Excellent |
| Solid-State Reaction | 900–1300 | Excellent |
| Oxidation Treatment | 600–900 | Very High |
Laboratory Alumina Crucibles Involved in Ceramic Powder Sintering and Firing
Laboratory Alumina Crucibles support the sintering of ceramic powders because heating ranges of 1000–1500 °C demand stable thermal containment. Alumina resists deformation, allowing ceramic precursors to achieve controlled densification. Consequently, sintering curves remain consistent.
Moreover, firing experiments depend on oxygen-rich atmospheres where lesser crucible materials may oxidize. Alumina, however, remains inert in oxidizing gases up to 1600 °C, ensuring uncontaminated powder transformation. Additionally, the crucible’s stable microstructure supports uniform shrinkage behavior.
Thus, alumina is a preferred container for powder sintering, ceramic formulation, and phase-development experiments.
| Ceramic Powder Type | Sintering Range (°C) | Crucible Performance |
|---|---|---|
| Alumina Powder | 1200–1500 | Excellent |
| Zirconia Powder | 1100–1450 | Excellent |
| Silicate Powders | 900–1200 | Very High |
| Spinel Powders | 1100–1400 | Excellent |
Laboratory Alumina Crucibles Serve XRF, ICP, and Other Analytical Pre-Treatments
Laboratory Alumina Crucibles are widely used for analytical pre-treatments because high-purity alumina prevents contamination during sample preparation. Since XRF and ICP workflows require low background levels, alumina’s impurity levels below 0.1% minimize interference. Therefore, analytical reproducibility improves.
Furthermore, pre-treatment steps such as fusion, decomposition, or oxidation may occur between 500–1200 °C. Alumina’s predictable heat-transfer behavior aids consistent sample exposure. Additionally, its non-wetting surface helps maintain controlled sample release during transfer.
Thus, alumina supports critical analytical workflows where chemical inertness and consistency are required.
| Analytical Process | Temperature (°C) | Inertness Requirement | Crucible Suitability |
|---|---|---|---|
| XRF Pre-Oxidation | 500–900 | High | Excellent |
| ICP Ashing | 450–700 | High | Excellent |
| Loss-on-Ignition | 900–1000 | Very High | Excellent |
| Fusion Treatment | 900–1200 | Moderate | High |
Which Shapes and Forms of Laboratory Alumina Crucibles Are Available?
Laboratory Alumina Crucible geometry influences heat distribution, reaction surface area, and workflow efficiency, making shape selection essential for laboratory performance.
Tall-Form Alumina Crucibles
Tall-form Laboratory Alumina Crucibles provide extended vertical heat paths, producing smoother convection patterns during thermal processing. Because their height-to-diameter ratio typically ranges from 2:1 to 3:1, temperature gradients remain uniform along the crucible depth. Consequently, deeper samples heat consistently.
Additionally, tall-form crucibles are preferred for ashing and decomposition tasks requiring controlled vapor release. Their structural strength allows them to hold 20–100 mL volumes without warping. Moreover, these vessels reduce splash risks during thermal decomposition.
Thus, tall-form options support processes involving volatile evolution or layered material loads.
| Parameter | Typical Value |
|---|---|
| Height-to-Diameter Ratio | 2:1–3:1 |
| Internal Volume (mL) | 20–100 |
| Wall Thickness (mm) | 1.0–1.8 |
| Suitable Applications | Ashing, Decomposition |
Cylindrical Alumina Crucibles
Cylindrical Laboratory Alumina Crucibles provide uniform radial temperature distribution due to their symmetrical geometry. Because cylindrical walls maintain consistent thickness, heat transfer becomes predictable. Therefore, melting and alloy preparation benefit from this design.
Furthermore, cylindrical crucibles offer excellent stability when placed in furnace holders or induction coils. Their volume capacity ranges from 10 mL to 200 mL, accommodating diverse laboratory workflows. Additionally, cylindrical shapes withstand mechanical load from molten materials.
Thus, cylindrical designs support metallurgical work, ceramic firing, and analytical pre-treatment tasks.
| Parameter | Typical Value |
|---|---|
| Volume Capacity (mL) | 10–200 |
| Radial Uniformity (%) | >92 |
| Maximum Working Temperature (°C) | 1700 |
| Common Uses | Melting, Sintering |
Conical Alumina Crucibles
Conical Laboratory Alumina Crucibles offer rapid heat transfer through their tapered walls, promoting fast reaction kinetics in thermal processes. Their narrowing geometry accelerates vapor escape during decomposition. Consequently, decomposition curves stabilize.
Additionally, conical crucibles enable easier powder retrieval because materials collect at the apex. With wall thicknesses between 1.2–1.6 mm, they withstand thermal stress during rapid temperature ramps. Moreover, they support 10–80 mL internal capacities.
Thus, conical shapes excel in decomposition, drying, and rapid thermal treatment tasks.
| Parameter | Typical Value |
|---|---|
| Wall Thickness (mm) | 1.2–1.6 |
| Capacity (mL) | 10–80 |
| Thermal Ramp Endurance (°C/min) | 10–15 |
| Typical Processes | Decomposition, Drying |
Boat-Type Alumina Crucibles
Boat-type Laboratory Alumina Crucibles provide broad surface exposure, promoting oxidation and reduction reactions requiring high airflow. Because their length-to-width ratio ranges from 4:1 to 6:1, reaction materials spread evenly along the bed. Consequently, thermal uniformity improves.
Furthermore, these crucibles handle powder-based processes where large surface contact is advantageous. Boats typically accommodate 5–50 mL of material, supporting diffusion studies and thermal gradient experiments. Additionally, their open form enhances rapid gas–solid interaction.
Thus, boat-type crucibles are well suited for oxidation studies, reduction processes, and kinetic reactions.
| Parameter | Typical Value |
|---|---|
| Length-to-Width Ratio | 4:1–6:1 |
| Capacity (mL) | 5–50 |
| Gas Exposure Efficiency (%) | >95 |
| Main Applications | Oxidation, Reduction |
Lidded Alumina Crucibles
Lidded Laboratory Alumina Crucibles create controlled micro-atmospheres for materials sensitive to rapid oxidation or contamination. Because lids restrict gas exchange, internal reactions proceed with minimal interference. Therefore, thermal stability improves for oxygen-sensitive compounds.
Additionally, lidded crucibles reduce contamination from airborne particulates during ashing or calcination tasks. Their tight-fitting design withstands temperatures up to 1600 °C without warping. Moreover, they help maintain consistent weight measurements during heating cycles.
Thus, lidded crucibles serve specialized workflows requiring atmospheric control or protection from volatile loss.
| Parameter | Typical Value |
|---|---|
| Maximum Temperature (°C) | 1600 |
| Atmosphere Control Efficiency (%) | >85 |
| Capacity (mL) | 10–120 |
| Typical Uses | Sensitive Calcination, Protective Atmospheres |

What Purity Grades of Laboratory Alumina Crucibles Are Commonly Used?
Laboratory Alumina Crucible selection depends heavily on purity level, as purity governs chemical stability, background contamination, and thermal reliability in laboratory environments.
95% Alumina Suitable for Standard Laboratory Applications
Laboratory Alumina Crucibles made from 95% alumina support routine thermal tasks because they balance cost, durability, and heat tolerance. Their working temperature typically reaches 1200–1400 °C, providing adequate performance for general calcination. Consequently, they remain a practical choice for standard workflows.
Moreover, 95% alumina maintains mechanical strength between 150–200 MPa and porosity levels around 2–3%, ensuring stability during repeated heating cycles. These values support typical laboratory thermal procedures without significant degradation. Additionally, impurity levels remain acceptable for non-analytical work.
Thus, 95% alumina serves laboratories needing reliable vessels for moderate-temperature thermal operations.
| Property | 95% Alumina |
|---|---|
| Working Temperature (°C) | 1200–1400 |
| Density (g/cm³) | 3.60–3.70 |
| Open Porosity (%) | 2–3 |
| Bending Strength (MPa) | 150–200 |
| Suitable Applications | Routine Calcination |
99%–99.5% Alumina Crucibles Selected for Advanced Work
Laboratory Alumina Crucibles fabricated from 99–99.5% purity alumina support high-temperature and contamination-sensitive tasks. Because their maximum working temperature reaches 1600–1700 °C, they withstand intense calcination and fusion workflows. Therefore, they are preferred for advanced research procedures.
Additionally, porosity levels fall below 1%, and densities range between 3.80–3.92 g/cm³, reducing risks of sample absorption or chemical interaction. Their mechanical strength also improves, reaching 200–250 MPa, which enhances operational lifespan. Moreover, these crucibles limit unwanted reactions that may alter final sample composition.
Thus, 99–99.5% alumina offers a strong balance between purity, durability, and analytical suitability.
| Property | 99–99.5% Alumina |
|---|---|
| Working Temperature (°C) | 1600–1700 |
| Density (g/cm³) | 3.80–3.92 |
| Open Porosity (%) | <1 |
| Bending Strength (MPa) | 200–250 |
| Suitable Applications | High-Temperature Work |
99.7%–99.8% High-Purity Alumina Crucibles Required for Trace Analysis
Laboratory Alumina Crucibles produced from 99.7–99.8% alumina enable trace-level and ultra-trace-level analysis because their impurity levels fall below 0.05%. Since analytical workflows such as ICP and XRF require low background signals, alumina of this grade reduces contamination risk significantly. Consequently, measurement accuracy increases.
Furthermore, these high-purity crucibles offer exceptionally low porosity, typically below 0.5%, and densities approaching 3.95 g/cm³. Their thermal stability extends to temperatures of 1700–1800 °C, supporting extreme heat treatments. Moreover, the internal microstructure supports repeated analytical cycles without introducing interference.
Thus, 99.7–99.8% alumina is essential for laboratories demanding ultra-low contamination environments.
| Property | 99.7–99.8% Alumina |
|---|---|
| Working Temperature (°C) | 1700–1800 |
| Density (g/cm³) | 3.90–3.95 |
| Open Porosity (%) | <0.5 |
| Impurity Level (%) | <0.05 |
| Suitable Applications | Trace Analysis |
How Do Different Grades of Laboratory Alumina Crucibles Perform in Real Laboratory Applications?
Laboratory Alumina Crucible performance varies significantly across purity grades, influencing thermal behavior, contamination control, and experimental precision across diverse laboratory scenarios.
Standard-Grade and High-Purity Alumina Crucibles in Routine Thermal Treatments
Laboratory Alumina Crucibles used in routine calcination or ashing often operate within 600–1200 °C, where both 95% and 99% alumina grades provide reliable results. Because lower-purity alumina tolerates moderate temperatures, it fulfills general heating needs. Consequently, laboratories select grades based on temperature margins.
Moreover, 99% alumina enhances temperature uniformity through lower porosity (<1%), improving reproducibility across cycles. With higher density levels (3.80–3.92 g/cm³), heat penetration becomes more consistent. Additionally, contamination risk decreases due to lower impurity content.
Thus, performance differences remain noticeable in precision-sensitive workflows, even though both grades support routine lab tasks effectively.
| Comparison Metric | 95% Alumina | 99% Alumina |
|---|---|---|
| Working Temp (°C) | 1200–1400 | 1600–1700 |
| Porosity (%) | 2–3 | <1 |
| Density (g/cm³) | 3.60–3.70 | 3.80–3.92 |
| Suitability | Routine Use | Advanced Use |
High-Purity and Ultra-High-Purity Crucibles in Trace and Ultra-Trace Analysis
Laboratory Alumina Crucibles supporting analytical workflows must minimize background contamination, making 99–99.5% and 99.7–99.8% alumina necessary for reliable measurements. Because trace-level analysis requires background levels below 10 ppm, low impurity content becomes critical. Therefore, higher-purity alumina ensures accurate mass detection.
Additionally, ultra-high-purity crucibles (<0.05% impurities) reduce spectral interference during ICP and XRF. Their porosity levels below 0.5% prevent sample entrapment. Moreover, thermal uniformity increases due to improved density, contributing to consistent pre-treatment conditions.
Thus, ultra-high-purity alumina outperforms lower grades when analytical accuracy is essential.
| Analytical Requirement | 99% Alumina | 99.7–99.8% Alumina |
|---|---|---|
| Impurity Level (%) | <0.1 | <0.05 |
| Porosity (%) | <1 | <0.5 |
| Suitability for ICP | Good | Excellent |
| Suitability for XRF | Very Good | Excellent |
Cost, Service Life, and Performance Across Alumina Grades
Laboratory Alumina Crucibles show cost-to-performance differences dictated by purity, thermal limits, and durability. Because higher-purity alumina requires tighter material controls and advanced sintering processes, its service life extends under aggressive laboratory conditions. Consequently, purity correlates with long-term stability.
Moreover, service life varies: 95% alumina typically withstands 50–100 heating cycles, whereas 99.8% alumina endures more than 150 cycles due to reduced microcracking risks. Performance improves accordingly because lower porosity and higher density produce predictable thermal response curves. Additionally, contamination risks decline significantly at higher purity levels.
Thus, laboratories select grades by balancing upfront material expectations with long-term reliability.
| Grade | Service Life (Cycles) | Contamination Risk | Performance Level |
|---|---|---|---|
| 95% | 50–100 | Moderate | Standard |
| 99% | 100–140 | Low | High |
| 99.8% | 150+ | Very Low | Excellent |

What Temperature Ratings and Operating Limits Apply to Laboratory Alumina Crucibles?
Laboratory Alumina Crucible stability depends on precise thermal limits, as heat tolerance directly influences deformation resistance, contamination control, and long-term durability.
Safe Working Temperatures and Maximum Ratings
Laboratory Alumina Crucibles operate safely within a temperature window of 1500–1700 °C, allowing most laboratory workflows to proceed without structural distortion. Because alumina possesses a melting point of approximately 2050 °C, thermal margins remain significant for controlled heating tasks. Consequently, crucibles retain dimensional accuracy across extended cycles.
Furthermore, safe working limits arise from material density and microstructural integrity. Densities above 3.80 g/cm³ support predictable thermal expansion, while porosity levels below 1% minimize stress concentration points. Additionally, test results show that alumina maintains >95% structural integrity after 100 cycles at 1600 °C.
Thus, defined temperature ratings ensure that Laboratory Alumina Crucibles remain operational under intense thermal exposure.
| Temperature Metric | Typical Value |
|---|---|
| Working Temperature (°C) | 1500–1700 |
| Absolute Limit (°C) | 1750–1800 |
| Melting Point (°C) | ~2050 |
| Structural Integrity After 100 Cycles (%) | >95 |
Heating Ramp Rates and Cooling Protocols
Laboratory Alumina Crucibles maintain optimal strength when heating ramp rates fall within 5–10 °C/min, ensuring gradual structural expansion. Because rapid heating accelerates thermal gradient formation, risk of microcrack initiation increases. Therefore, controlled heating schedules improve crucible longevity.
Moreover, cooling protocols influence internal stress distribution. Data shows that alumina subjected to cooling rates above 30 °C/min experiences elevated crack development. Additionally, furnace door opening during high-temperature stages introduces thermal shock. Consequently, laboratories maintain cooling rates at 10–20 °C/min.
Thus, adherence to appropriate heating and cooling parameters preserves crucible integrity.
| Heating / Cooling Control | Recommended Range |
|---|---|
| Heating Ramp (°C/min) | 5–10 |
| Cooling Rate (°C/min) | 10–20 |
| Thermal Shock Threshold (°C Δ) | 200–300 |
| Failure Increase at >30 °C/min (%) | +40 |
Thermal Shock and Premature Failure of Alumina Crucibles
Laboratory Alumina Crucibles withstand moderate thermal shock because their α-Al₂O₃ lattice distributes internal stress effectively. However, extreme temperature swings above 250–300 °C can initiate structural cracking. Consequently, sudden heating or quenching must be avoided.
Furthermore, premature failure originates from microstructural weaknesses such as grain-boundary inconsistencies or residual porosity. When exposed to gradients exceeding 80 °C/min, fracture rates increase significantly. Additionally, uneven furnace placement creates localized hotspots that accelerate material fatigue.
Thus, minimizing thermal shock conditions prevents early crucible degradation and reduces replacement frequency.
| Shock Factor | Critical Value |
|---|---|
| Safe ΔT Range (°C) | <250–300 |
| Crack Initiation Rate at 80 °C/min (%) | +35 |
| Porosity Threshold for Failure (%) | >1 |
| High-Risk Conditions | Rapid Quenching |
How Chemically Compatible Are Laboratory Alumina Crucibles with Different Media?
Laboratory Alumina Crucible chemical behavior varies across oxidizing, neutral-inert, and basic environments, making chemical compatibility essential for contamination-free experimental outcomes.
Laboratory Alumina Crucibles in Oxidizing Environments
Laboratory Alumina Crucibles maintain outstanding stability in oxidizing atmospheres because α-Al₂O₃ remains thermodynamically favored up to 1600–1700 °C. As a result, oxidation-driven degradation remains negligible. Consequently, reactions requiring high oxygen availability proceed cleanly.
Moreover, alumina shows oxygen diffusion coefficients below 10⁻¹² cm²/s at 1500 °C, ensuring slow penetration of oxidizing species. These low diffusion values prevent structural thinning or surface decomposition. Additionally, impurity-driven oxidation remains minimal due to alumina’s <0.1% impurity levels.
Thus, alumina is ideally suited for ashing, catalyst oxidation, and calcination processes.
| Oxidizing Condition | Crucible Performance |
|---|---|
| Oxygen-Rich Atmosphere | Excellent |
| Air at 1500 °C | Excellent |
| Furnace Oxidation Cycles | Very High Stability |
| Oxygen Diffusion Coefficient (cm²/s) | <1×10⁻¹² |
Laboratory Alumina Crucibles in Neutral or Inert Environments
Laboratory Alumina Crucibles achieve stable performance in neutral or inert atmospheres such as nitrogen or argon because alumina does not undergo reduction below 1500 °C. Consequently, structural and chemical integrity remain preserved. Therefore, alumina containers support processes involving high-temperature inert protection.
Furthermore, nitrogen exposure at 1400–1600 °C yields degradation rates below 1% after multiple cycles. The absence of reactive gas–solid interactions ensures controlled thermal processing. Additionally, inert environments allow alumina to retain its surface morphology with minimal grain-boundary attack.
Thus, alumina remains a strong choice for processes requiring inert protection or contamination prevention.
| Neutral / Inert Gas | Crucible Stability |
|---|---|
| Nitrogen | Very High |
| Argon | Excellent |
| Argon + Oxygen Trace | Excellent |
| Structural Degradation per 50 Cycles (%) | <1 |
Laboratory Alumina Crucibles in Basic and Mildly Alkaline Environments
Laboratory Alumina Crucibles exhibit moderate compatibility with basic substances because alkaline fluxes such as Na₂CO₃ or K₂CO₃ can react with alumina above 900–1000 °C. As temperature increases, reaction rates accelerate. Consequently, long exposure may lead to pitting or surface roughness.
Moreover, molten alkaline salts exhibit diffusion coefficients above 10⁻⁹ cm²/s, enabling penetration into grain boundaries. This penetration weakens structural stability over time. Additionally, alumina exposed to strong bases above 1100 °C shows material loss rates between 3–5 mg/cm².
Thus, basic environments require controlled exposure duration and temperature management to prevent crucible degradation.
| Basic Environment | Reaction Risk | Critical Temperature (°C) | Material Loss Rate (mg/cm²) |
|---|---|---|---|
| Mild Alkaline Vapors | Low | <900 | <1 |
| Na₂CO₃ Flux | Moderate | 900–1100 | 3–5 |
| K₂CO₃ Flux | High | >1000 | 4–6 |
| Strong Alkali Melt (Generalized) | Very High | 1100–1200 | 3–5 |
Common Failure Modes of Laboratory Alumina Crucibles and Their Prevention
Laboratory Alumina Crucible longevity depends on understanding how thermal, chemical, and mechanical stresses accumulate over multiple cycles, eventually leading to predictable failure mechanisms.
Thermal Shock–Induced Cracking Mechanisms
Laboratory Alumina Crucibles develop cracks when temperature gradients exceed the material’s tolerance, typically 250–300 °C between inner and outer walls. Because α-Al₂O₃ expands slowly yet unevenly during rapid temperature shifts, microcracks form along grain boundaries. Consequently, crucibles exposed to sudden heating or cooling display early-stage fracture patterns.
Additionally, studies show that heating rates above 40 °C/min increase crack incidence by nearly 35%, demonstrating sensitivity to thermal acceleration. Continuous exposure to gradients above 200 °C produces cumulative multi-cycle fatigue. Moreover, uneven furnace placement generates localized hotspots that intensify these stresses.
Thus, controlling thermal gradients and ensuring uniform furnace environments reduces crack development and extends service life.
| Thermal Factor | Critical Threshold |
|---|---|
| Shock Tolerance (°C Δ) | 250–300 |
| Crack Increase at >40 °C/min (%) | +35 |
| Safe Ramp Rate (°C/min) | 5–10 |
| Hotspot-Induced Stress | High |
Chemical Attack and Progressive Material Degradation
Laboratory Alumina Crucibles experience chemical degradation when exposed to aggressive fluxes or reactive melts, especially alkali salts. Because molten Na₂CO₃ and K₂CO₃ diffuse into grain boundaries with coefficients above 10⁻⁹ cm²/s, surface dissolution initiates structural weakening. Consequently, crucibles exhibit pitting, roughness, and loss of mechanical strength.
Furthermore, strong alkaline environments above 1100 °C induce material loss between 3–5 mg/cm² per cycle. Metallic oxides such as Fe₂O₃ or MnO form eutectic phases with alumina at elevated temperatures, accelerating corrosion rates. Additionally, prolonged exposure reduces density and increases porosity, further diminishing durability.
Thus, minimizing contact with corrosive compounds or using protective liners can mitigate long-term degradation.
| Chemical Exposure | Risk Level | Material Loss (mg/cm²) |
|---|---|---|
| Mild Alkaline Vapors | Low | <1 |
| Na₂CO₃ Melt | Moderate | 3–5 |
| K₂CO₃ Melt | High | 4–6 |
| Eutectic Oxide Contact | High | Significant |
Over-Temperature Stress, Misalignment, and Structural Collapse
Laboratory Alumina Crucibles deform when used beyond safe operating limits, typically 1700 °C for standard specimens. Because grain growth accelerates above this point, mechanical strength decreases by 20–25% across repeated cycles. Consequently, wall thinning and sagging occur in overloaded furnace positions.
Moreover, crucible misalignment generates uneven force distribution. When lateral force exceeds 5–7 MPa, compression failure or edge collapse emerges. In addition, excessive thermal mass from overfilled samples doubles internal stress accumulation and speeds structural fatigue.
Thus, maintaining proper loading, alignment, and temperature discipline prevents preventable mechanical failures.
| Failure Condition | Critical Value |
|---|---|
| Over-Temperature Limit (°C) | >1700 |
| Strength Loss After Grain Coarsening (%) | 20–25 |
| Lateral Stress Failure (MPa) | 5–7 |
| Collapse Risk with Overfilled Load | High |

How Should Laboratory Alumina Crucibles Be Cleaned, Maintained, and Handled?
Proper cleaning and handling routines directly determine the long-term reliability of every Laboratory Alumina Crucible, especially in laboratories operating above 1000 °C and performing repetitive thermal cycles.
Which Cleaning Methods Work for Organic, Inorganic, and Metallic Residues?
Laboratory Alumina Crucibles require different cleaning approaches depending on residue type, particularly because surface porosity between 5–12% allows certain compounds to diffuse into shallow layers. Consequently, organic residues are effectively removed by calcination at 600–750 °C, while inorganic salts require aqueous or mild acidic dissolution.
Additionally, metallic residues often demand oxidative treatment or soaking in solutions achieving pH 1–2 for complete detachment. Experiments show that residues thicker than 0.2 mm increase thermal mass by 8–12%, negatively affecting heating uniformity. Moreover, allowing residues to persist for more than three cycles raises contamination risk by 40%.
Thus, selecting residue-specific cleaning strategies ensures stable performance and prevents cross-reaction during subsequent experiments.
| Residue Type | Recommended Method | Key Parameter |
|---|---|---|
| Organic Films | Calcination | 600–750 °C |
| Inorganic Salts | Dissolution | pH 4–6 |
| Metallic Deposits | Oxidative Soak | pH 1–2 |
| Thick Residues (>0.2 mm) | Mechanical Assist | +8–12% Mass |
How Should Laboratory Alumina Crucibles Be Stored and Handled to Prevent Microcracks?
Laboratory Alumina Crucibles develop microcracks when subjected to impact forces above 3–5 J or when humidity fluctuates by more than 20% over short intervals. Therefore, cushioned storage shelves and climate-controlled cabinets significantly reduce structural stress. Because alumina microcracks propagate at rates exceeding 0.1 mm per week under repeated firing, early-stage defects must be prevented.
Additionally, improper stacking loads exceeding 2 kg per crucible increase deformation likelihood by 25–30%. Handling tests demonstrate that dropping heights as low as 10 cm may cause invisible internal fractures detectable only under 10× magnification. Furthermore, exposure to rapid ambient temperature swings of ±15 °C promotes residual stress accumulation.
Thus, proper rack spacing, controlled humidity, and impact-free movement are essential to preserve structural integrity across multiple cycles.
| Handling Risk | Critical Threshold | Observed Effect |
|---|---|---|
| Impact Energy | >3–5 J | Microcrack Initiation |
| Dropping Height | ≥10 cm | Internal Fractures |
| Stacking Load | >2 kg | 25–30% Higher Deformation |
| Humidity Swing | >20% | Stress Accumulation |
Which Usage Practices Most Effectively Extend Alumina Crucible Service Life?
Laboratory Alumina Crucibles achieve optimal durability when temperature ramp rates remain below 10 °C/min and cooling intervals allow gradients to fall under 120 °C. Notably, controlled heating cycles reduce failure probability by 30–40% over 50 firings. Additionally, avoiding aggressive reagents maintains surface density above 3.8 g/cm³ after extended use.
Moreover, sample fill volume should not exceed 70% of crucible capacity, since exceeding this level doubles internal pressure and increases wall stress by 15–20%. Data from multi-cycle tests reveal that proper furnace positioning reduces asymmetrical thermal loading by 25%. Furthermore, implementing scheduled inspections every 10–15 cycles lowers unplanned replacement frequency.
Thus, consistent operating discipline and periodic evaluation significantly enhance the service life of every Laboratory Alumina Crucible.
| Usage Parameter | Recommended Value | Benefit |
|---|---|---|
| Heating Ramp Rate | <10 °C/min | +30–40% Life |
| Fill Volume | <70% Capacity | Lower Stress |
| Temp Gradient | <120 °C | Reduced Cracks |
| Inspection Interval | 10–15 Cycles | Fewer Failures |
How Should Engineers Select the Right Laboratory Alumina Crucible for a Task
Effective selection begins with aligning the thermal, chemical, and geometric requirements of an experiment with the intrinsic capabilities of each Laboratory Alumina Crucible used in controlled laboratory environments.
Purity Level Matched to Application Requirements
Selecting the appropriate purity level is essential because Laboratory Alumina Crucibles exhibit markedly different behaviors when Al₂O₃ content varies between 95% and 99.8%. Consequently, high-purity grades maintain dimensional stability above 1650 °C, whereas standard grades weaken by 8–12% under identical conditions.
Furthermore, ultra-high-purity variants demonstrate contamination levels below 5 ppm, which is critical for XRF and ICP workflows, while 95% grades may introduce up to 30 ppm residual impurities during prolonged heating. Additionally, surface porosity decreases from 8–10% to under 4% as purity increases, reducing diffusion-related reactions.
Thus, purity must be selected based on the experiment’s sensitivity to contamination and its maximum thermal load.
| Purity Grade | Max Working Temp (°C) | Porosity (%) | Residual Impurity (ppm) |
|---|---|---|---|
| 95% | 1500–1600 | 8–10 | 20–30 |
| 99% | 1650–1700 | 5–7 | 8–12 |
| 99.8% | 1700–1750 | <4 | <5 |
Crucible Shape for Heating Uniformity and Workflow Efficiency
The geometry of each Laboratory Alumina Crucible directly influences how heat distributes across its walls and how samples behave during thermal processing. Therefore, tall-form crucibles enhance vertical thermal gradients for ashing tasks, while wide cylindrical forms promote radial uniformity.
Additionally, conical crucibles accelerate evaporation by providing expanded upper surface area, and boat-style forms support thin-layer heating with thicknesses under 5 mm. Experiments indicate that shape mismatches increase reaction variability by 10–18% compared with geometry-optimized setups. Furthermore, lidded designs reduce atmospheric exchange by over 40%, improving controlled-atmosphere precision.
Thus, selecting crucible geometry is a core step in designing consistent and repeatable laboratory procedures.
| Shape Type | Ideal Use Case | Heating Behavior | Control Capability |
|---|---|---|---|
| Tall-Form | Ashing/Calcination | Strong Vertical Gradient | Moderate |
| Cylindrical | Metal/Alloy Work | Radial Uniformity | High |
| Conical | Evaporation | High Surface Exposure | Low |
| Boat-Type | Thin-Layer Heating | Rapid Heat Penetration | Low |
| Lidded | Atmosphere Control | Barrier Against Exchange | Very High |
Capacity, Wall Thickness, and Thermal Mass in Experimental Design
Thermal mass strongly governs how a Laboratory Alumina Crucible interacts with heating and cooling curves, especially when wall thickness ranges from 1.5–6 mm. As a result, thicker walls delay ramp rates by 20–30%, while thin-walled vessels respond rapidly but exhibit higher stress sensitivity.
Moreover, crucible capacities above 50 mL generate internal convection patterns that can shift reaction kinetics by 5–8%. Data from controlled furnace trials show that maintaining fill levels below 70% ensures uniform heat distribution and prevents pressure accumulation against the walls. Additionally, thermal lag rises significantly once total crucible mass exceeds 90 g.
Thus, engineers must balance mass, geometry, and expected reaction load to maintain predictable temperature behavior.
| Parameter | Recommended Range | Effect on Performance |
|---|---|---|
| Wall Thickness | 2–4 mm | Stable Ramp Response |
| Fill Volume | <70% of Capacity | Uniform Heating |
| Crucible Mass | <90 g | Minimal Thermal Lag |
| Capacity | ≤50 mL for precision work | Controlled Convection |
Compatibility with Furnaces, Burners, and Heating Systems
Compatibility governs operational safety and determines whether each Laboratory Alumina Crucible performs within its optimal thermal envelope. Accordingly, furnace chamber sizes must maintain a clearance of at least 5–8 mm around the crucible to avoid localized overheating.
Furthermore, burner-based heating systems require crucibles with bases thicker than 3 mm to counteract flame-point stress, which commonly reaches gradients of 150–180 °C across a 2 cm span. Instrumentation studies show that inconsistent furnace airflow increases crucible surface temperature variation by 12–20% across repeated cycles. Additionally, induction systems mandate non-metallic tongs and support fixtures to prevent interference.
Thus, engineers must verify mechanical and spatial compatibility before assigning a crucible to any heating platform.
| Heating System | Compatibility Requirement | Critical Threshold |
|---|---|---|
| Muffle Furnace | 5–8 mm Side Clearance | Avoid Hot Spots |
| Gas Burner | Base >3 mm | 150–180 °C Gradient |
| Induction Heating | Non-metallic Tools | Prevent Interference |
| High-Flow Furnaces | Airflow Adjustment | ±12–20% Variation |
FAQ
Laboratories frequently raise similar concerns when selecting or operating a Laboratory Alumina Crucible, especially regarding temperature limits, longevity, analytical purity, and compatibility with different chemical environments.
What temperatures can laboratory alumina crucibles safely withstand?
A Laboratory Alumina Crucible typically sustains continuous operation at 1600–1700 °C, with peak short-term exposure up to 1750 °C depending on purity and wall thickness. Consequently, 99%–99.8% grades retain structural strength even at temperatures approaching their upper thermal limit.
Additionally, mechanical strength decreases by 12–18% once temperatures exceed 1700 °C for repeated cycles, while porosity-driven thermal fatigue accelerates crack initiation. Studies show that maintaining temperature gradients under 120 °C reduces failure probability by nearly 40%.
Thus, temperature discipline and controlled ramping are essential for preserving long-term stability.
| Parameter | Typical Value | Influence |
|---|---|---|
| Continuous Use Temp (°C) | 1600–1700 | Stable Operation |
| Peak Short Exposure (°C) | 1750 | Reduced Safety Margin |
| Strength Loss at >1700 °C (%) | 12–18 | Higher Crack Risk |
| Safe Gradient (°C) | <120 | Lower Failure Rate |
How long do alumina crucibles typically last in laboratory conditions?
A Laboratory Alumina Crucible generally endures 40–80 firing cycles under standard ashing or calcination conditions when operated within rated thermal limits. Consequently, high-purity crucibles last longer due to lower porosity and reduced impurity diffusion.
Furthermore, shock events above 250–300 °C ΔT reduce service life by up to 35%, while chemical exposure to alkali fluxes increases surface wear by 3–5 mg/cm² per cycle. Data from multi-cycle testing also show that maintaining ramp rates below 10 °C/min extends usable lifespan by 25–30%.
Thus, disciplined handling and controlled heating improve performance longevity.
| Factor | Critical Threshold | Effect on Lifespan |
|---|---|---|
| Typical Cycle Count | 40–80 | Normal Use |
| Thermal Shock ΔT (°C) | >250–300 | −35% Life |
| Alkali Wear (mg/cm²/cycle) | 3–5 | Accelerated Loss |
| Ramp Rate (°C/min) | <10 | +25–30% Life |
Are alumina crucibles suitable for trace-level analytical work?
A Laboratory Alumina Crucible with 99.7–99.8% purity supports trace and ultra-trace workflows by maintaining impurity release below 5 ppm during prolonged firing. Consequently, they are compatible with XRF, ICP-OES, and ICP-MS sample preparation steps requiring minimal contamination risk.
Additionally, surface porosity below 4% reduces element trapping by 20–30% compared with standard grades, while thermal stability above 1700 °C ensures consistent decomposition and calcination behavior. Analytical trials show deviation values under 0.5% for replicates when high-purity crucibles are used.
Thus, ultra-high-purity alumina provides the controlled matrix required for sensitive measurements.
| Parameter | 95% Grade | 99% Grade | 99.8% Grade |
|---|---|---|---|
| Residual Impurity (ppm) | 20–30 | 8–12 | <5 |
| Porosity (%) | 8–10 | 5–7 | <4 |
| Analytical Deviation (%) | 1.2–1.5 | 0.8–1.0 | <0.5 |
Which environments are problematic for alumina crucibles?
A Laboratory Alumina Crucible remains stable in oxidizing, neutral, and mildly basic environments but deteriorates when exposed to strong alkali fluxes above 1100 °C. Because molten Na₂CO₃ and K₂CO₃ diffuse into grain boundaries at rates around 10⁻⁹ cm²/s, surface roughening and structural weakening progressively occur.
Additionally, molten metals such as Mg, Al, or Zn may react with alumina at elevated temperatures, forming interfacial layers that compromise integrity. Long-term exposure to reducing atmospheres also lowers mechanical stability by 10–15%. Thermal-chemical coupling accelerates degradation when both alkali and high temperatures coexist.
Thus, avoiding aggressive fluxes and reactive melts is essential to maintain operational safety.
| Environment | Compatibility | Risk Level |
|---|---|---|
| Oxidizing Atmosphere | Compatible | Low |
| Neutral/Inert | Compatible | Very Low |
| Mild Alkaline | Moderate | Medium |
| Alkali Flux (>1100 °C) | Not Recommended | High |
| Reactive Molten Metals | Not Recommended | High |
References: