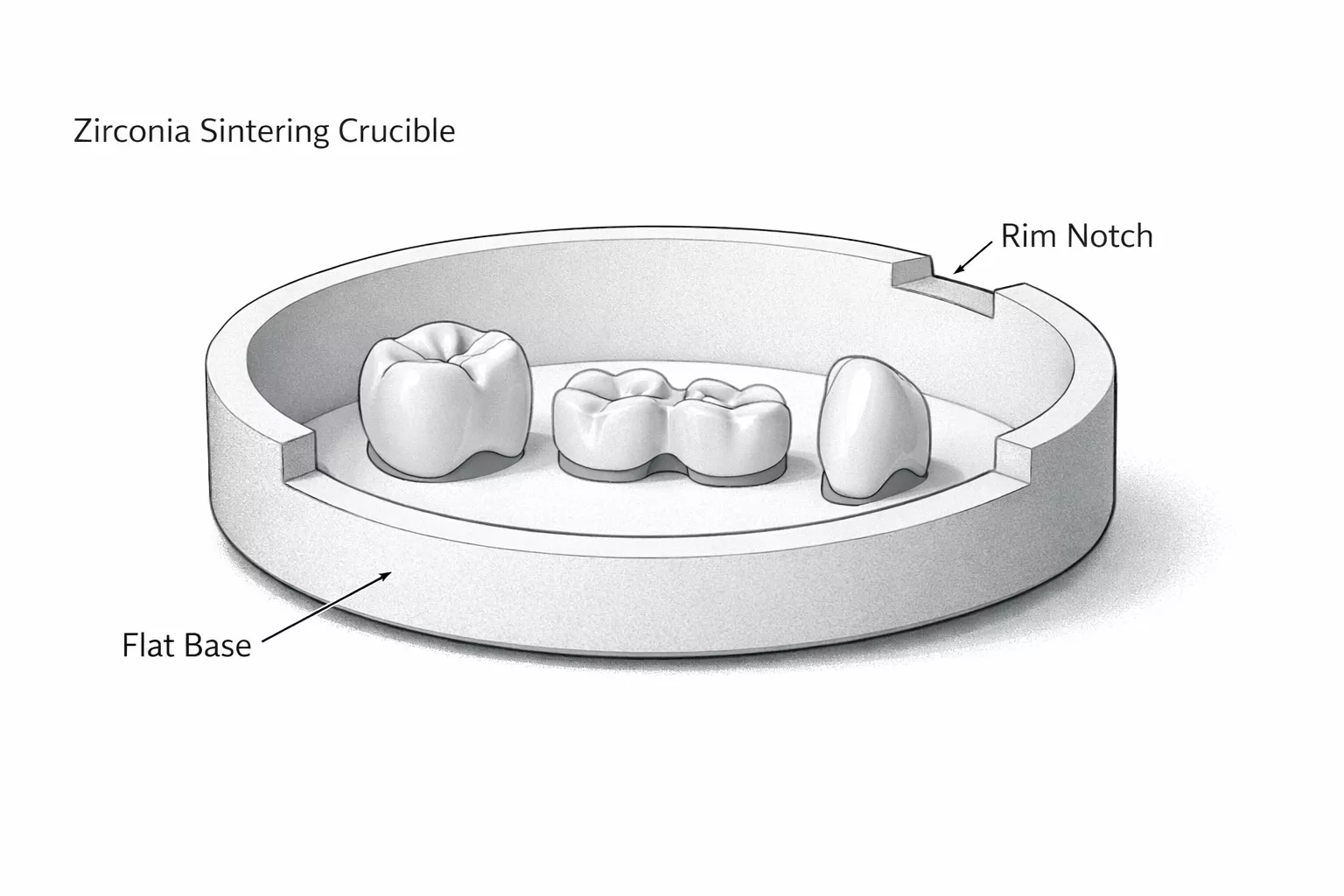
Instability in melting and casting rarely originates from peak temperature alone; instead, it emerges when thermal loads, phase behavior, and melt interactions exceed material coherence during coupled processes.
This article explains why zirconia crucibles maintain stability in melting and casting environments. Moreover, it demonstrates how crystal phase control, thermomechanical properties, and chemical compatibility jointly sustain performance across precious metal and superalloy applications.
Accordingly, the discussion establishes melting–casting thermal demands as a reference frame, then systematically connects zirconia material science to observed stability in real high-temperature operations. Ultimately, the goal is to provide an engineering basis for understanding why zirconia functions reliably under extreme metallurgical conditions.

Zirconia Crucible for Melting and Casting is frequently evaluated under conditions where melting and pouring operate as a single thermal event. Furthermore, stability must be assessed against gradients, dwell periods, and transient heat loss rather than furnace setpoints alone.
Melting and Casting Thermal Demands
Melting and casting impose a compound thermal environment characterized by sustained high temperatures and rapid transient changes1. Moreover, these processes generate spatial and temporal gradients that directly challenge ceramic phase stability. Consequently, understanding the thermal demand profile becomes essential before evaluating zirconia material behavior.
Temperature plateaus and gradients in melt holding
During melt holding, zirconia crucible walls experience prolonged exposure to elevated temperatures while maintaining contact with molten metals. In industrial precious metal processing, holding temperatures typically range from 1450–1750 °C, whereas superalloy melting frequently extends to 1550–1700 °C, depending on alloy chemistry and furnace design.
Despite apparently stable setpoints, internal temperature gradients commonly develop across the crucible wall. For example, measured radial gradients of 40–180 °C are routinely observed between the inner hot face and the outer shell during steady-state holding. Furthermore, vertical gradients arise because electromagnetic stirring or natural convection concentrates thermal energy near the melt surface. As a result, zirconia must sustain phase stability and mechanical integrity under non-uniform thermal fields rather than uniform heating assumptions.
These gradients explain why stability cannot be inferred solely from maximum service temperature. Instead, the ability of zirconia to accommodate differential expansion without generating excessive internal stress becomes a defining factor during melt holding.
Thermal cycling severity across successive heats
Repeated melting cycles amplify thermal demands beyond single-use evaluations. Notably, production casting environments often subject crucibles to 20–80 heating cycles per month, while laboratory alloy development may involve 10–40 cycles with wider temperature swings.
Each cycle introduces expansion and contraction strains that accumulate within the ceramic lattice. Experimental observations show that when heating and cooling rates vary by more than ±20 % between cycles, internal stress redistribution accelerates microstructural fatigue. Moreover, sustained cycling at temperatures above 1500 °C promotes gradual creep relaxation in zirconia, altering stress balance over time. Consequently, stability must be defined as resistance to progressive degradation rather than immediate fracture.
Therefore, zirconia crucibles are evaluated not only by their survival of initial melts, but also by their ability to maintain dimensional and structural consistency across extended cycling histories.
Pouring induced transient cooling and reheating
The transition from melt holding to pouring introduces the most abrupt thermal disturbance in casting operations. During pouring, molten metal exits the crucible within 5–20 seconds, exposing the inner surface to rapid radiative and convective cooling.
Thermal imaging studies indicate that rim and lip regions can experience localized cooling rates exceeding 8–15 °C/s, while the lower wall remains near holding temperature. Subsequently, residual melt or furnace radiation can partially reheat exposed areas within 30–90 seconds, creating short-lived but severe thermal reversals. As an illustration, rim-to-midwall temperature differentials of 120–200 °C have been recorded immediately after pouring events.
Such transient conditions explain why zirconia stability is frequently validated at pouring transitions rather than during soaking. Accordingly, materials capable of preserving phase integrity and fracture resistance under rapid thermal fluctuation are favored in melting and casting systems.
Thermal demand characteristics across melting and casting stages
| Process stage | Typical temperature range (°C) | Dominant thermal feature | Gradient magnitude (°C) | Stability implication |
|---|---|---|---|---|
| Melt ramp and preheat | 800–1500 | Rapid expansion onset | 30–120 | Phase accommodation required |
| Melt holding | 1450–1750 | Sustained thermal load | 40–180 | Creep and stress balance |
| Pouring transition | 1200–1700 | Transient cooling/reheating | 80–200 | Crack resistance critical |
| Post-pour stabilization | 600–1200 | Reverse gradients | 40–160 | Fatigue resistance |

Chemical Compatibility With Molten Metals
Chemical stability defines whether a crucible preserves melt integrity over time. Moreover, compatibility depends on controlled interfacial reactions, oxygen potential2 balance, and limited mass transfer between ceramic and molten metal. Consequently, zirconia performance must be evaluated through thermodynamic and kinetic interactions rather than temperature resistance alone.
Wetting behavior and interfacial reactions
Wetting behavior governs the extent of contact between molten metal and the crucible surface. In precious metal systems, platinum-group melts typically exhibit contact angles above 120° on dense zirconia surfaces at 1500–1700 °C, indicating weak wetting and limited interfacial adhesion.
Under sustained holding, interfacial reactions are controlled by diffusion rather than chemical affinity. For instance, zirconia demonstrates reaction layer growth rates below 1–2 μm/hour when exposed to noble metal melts, provided surface density exceeds 98.5 % of theoretical density. Furthermore, limited wetting reduces capillary infiltration, which otherwise accelerates structural weakening at grain boundaries. As a result, stable interfacial behavior supports repeated melting cycles without progressive bonding or spalling.
Therefore, zirconia’s inherent resistance to wetting forms a primary mechanism that preserves crucible geometry and melt cleanliness during casting operations.
Oxygen potential and oxide stability in melts
Molten metals impose distinct oxygen chemical potentials that influence oxide stability. Zirconia remains thermodynamically stable across a wide oxygen partial pressure range, from 10⁻¹⁵ to 10⁻⁶ atm at temperatures above 1400 °C, which overlaps with conditions encountered in induction and resistance furnaces.
In nickel- and cobalt-based superalloy melts, dissolved oxygen activity fluctuates during alloying additions and degassing stages. Notably, zirconia does not undergo reduction or suboxide formation within these regimes, unlike certain alumina or silica-containing ceramics. Consequently, phase integrity is preserved even when oxygen activity shifts during melt conditioning. In addition, stabilized zirconia maintains lattice oxygen mobility without catastrophic vacancy collapse, preventing structural destabilization.
Thus, oxide stability under variable oxygen potentials explains why zirconia sustains chemical coherence in both precious metal and superalloy environments.
Contamination pathways and purity preservation
Contamination risk arises when crucible constituents dissolve or react with the melt. Zirconia exhibits exceptionally low solubility in noble and base metal melts, typically below 10⁻⁴ wt% under standard casting temperatures.
Experimental melt analyses reveal that zirconia-derived inclusions remain below detection limits of 5–10 ppm after multiple heats, provided impurity phases such as silica or alkali oxides are controlled below 0.1 wt% in the ceramic matrix. Moreover, the absence of glassy grain-boundary phases limits transport pathways for contaminant species. Consequently, melt purity is preserved across successive cycles without progressive chemical loading.
Accordingly, zirconia Crucible for Melting and Casting supports applications where compositional fidelity and defect suppression are critical performance metrics rather than secondary benefits.
Chemical interaction characteristics in melting and casting environments
| Interaction factor | Precious metal melts | Superalloy melts | Typical quantitative range | Stability implication |
|---|---|---|---|---|
| Wetting angle (°) | Weak wetting | Moderate wetting | 110–140 | Limits infiltration |
| Reaction layer growth (μm/h) | Minimal | Low | < 2 | Preserves interface |
| Oxygen potential tolerance (atm) | Wide | Variable | 10⁻¹⁵–10⁻⁶ | Phase stability |
| Elemental pickup (ppm) | Very low | Low | < 10 | Melt purity retention |
Zirconia Phase Stability Under Heat
Phase stability governs whether zirconia maintains coherence under prolonged thermal exposure. Moreover, melting and casting conditions activate phase-related stress mechanisms that differ fundamentally from short-term firing tests. Consequently, stabilized zirconia systems must be evaluated by how phase transformations interact with thermal gradients and mechanical constraints.
Phase transformations and stress generation
Pure zirconia undergoes a monoclinic–tetragonal transformation near 1170 °C, accompanied by a volumetric change of approximately 3–5 %. In melting and casting environments exceeding 1400 °C, this transformation would repeatedly activate during heating and cooling if left unstabilized.
Stabilized zirconia suppresses this transformation by retaining tetragonal or cubic phases across the operating range. Quantitative diffraction studies show that fully stabilized compositions maintain phase fractions above 95 % tetragonal/cubic after 100+ thermal cycles between ambient temperature and 1600 °C. Furthermore, suppression of abrupt phase change prevents localized tensile stress accumulation at grain boundaries. As a result, internal stress generation remains distributed rather than concentrated, enabling structural continuity under severe gradients.
Therefore, phase stabilization directly converts zirconia from a transformation-prone ceramic into a thermally resilient crucible material.
Transformation toughening and crack resistance
Transformation toughening provides an additional stability mechanism unique to partially stabilized zirconia. When a propagating crack introduces localized stress, retained metastable tetragonal grains can transform to the monoclinic phase, absorbing energy through controlled volumetric expansion.
Mechanical testing under high-temperature conditions demonstrates fracture toughness values of 6–10 MPa·m¹ᐟ² for partially stabilized zirconia at 1200–1400 °C, compared with 3–4 MPa·m¹ᐟ² for non-transforming oxide ceramics. Moreover, this toughening effect remains active even after extended thermal exposure, provided grain size remains below 0.5–0.8 μm. Consequently, crack growth is arrested before reaching critical dimensions during transient thermal shocks.
Thus, transformation toughening operates as an internal stress buffer, enhancing crack resistance without compromising chemical inertness.
High temperature creep tendencies and mitigation
At sustained melting temperatures, creep becomes a relevant deformation mechanism. Zirconia exhibits measurable creep above 1400 °C, with strain rates strongly dependent on grain size, stabilizer content, and applied stress.
Dense stabilized zirconia with grain sizes below 1 μm typically shows steady-state creep rates on the order of 10⁻⁷–10⁻⁸ s⁻¹ under compressive stresses of 5–10 MPa at 1500 °C. Furthermore, stabilizer selection influences vacancy mobility, which governs creep accommodation rather than brittle failure. Consequently, controlled creep allows gradual stress relaxation instead of catastrophic cracking during prolonged melt holding.
Therefore, phase-stable zirconia balances limited creep with structural rigidity, mitigating long-term deformation while preserving crucible geometry.
Phase stability indicators relevant to melting and casting
| Phase-related factor | Quantitative indicator | Typical operating range | Stability contribution |
|---|---|---|---|
| Tetragonal/cubic phase retention (%) | > 95 | Up to 1600 °C | Suppresses volumetric shock |
| Fracture toughness (MPa·m¹ᐟ²) | 6–10 | 1200–1400 °C | Crack resistance |
| Critical grain size (μm) | < 0.8 | High-temperature service | Maintains toughening |
| Creep rate (s⁻¹) | 10⁻⁸–10⁻⁷ | 1450–1550 °C | Stress relaxation |

Stabilizer Systems and Performance Classes
Stabilizer chemistry defines how zirconia maintains phase integrity under melting and casting conditions. Moreover, different stabilizer systems expand or constrain usable temperature windows, thermal cycling tolerance, and long-term stability. Consequently, understanding performance classes becomes essential for aligning zirconia behavior with metallurgical duty cycles.
Yttria stabilized zirconia performance window
Yttria stabilized zirconia is widely applied because yttrium ions effectively suppress monoclinic transformation across a broad temperature range. Typically, compositions containing 3–8 mol% Y₂O₃ retain tetragonal or cubic phases from ambient temperature up to 1600–1700 °C.
At melting temperatures, yttria stabilized zirconia exhibits stable lattice oxygen mobility without structural collapse. Mechanical characterization shows flexural strength values of 600–900 MPa at room temperature, decreasing gradually to 300–450 MPa near 1400 °C. Furthermore, thermal cycling tests exceeding 100 cycles between 25 °C and 1500 °C demonstrate minimal phase degradation when grain size remains controlled. Consequently, this system provides a wide and predictable operating window for both precious metal and superalloy casting.
Therefore, yttria stabilization is commonly selected where repeated heating cycles and moderate thermal gradients dominate service conditions.
Magnesia stabilized zirconia performance window
Magnesia stabilized zirconia employs MgO contents around 8–10 mol% to stabilize the cubic phase at elevated temperatures. This system demonstrates exceptional resistance to high-temperature creep and long-term dimensional drift.
Under continuous exposure at 1500–1700 °C, magnesia stabilized zirconia shows creep rates below 5 × 10⁻⁸ s⁻¹ at compressive stresses of 5 MPa. Moreover, its thermal conductivity is slightly higher than yttria stabilized variants, typically 2.5–3.0 W·m⁻¹·K⁻¹ at 1000 °C, which moderates internal gradients during extended melt holding. However, partial destabilization can occur during slow cooling through 800–1000 °C, requiring controlled cooldown protocols. As a result, this system excels in long soak applications with stable thermal programs.
Thus, magnesia stabilized zirconia aligns well with superalloy melting scenarios emphasizing sustained thermal load rather than rapid cycling.
Calcia stabilized zirconia performance window
Calcia stabilized zirconia uses CaO levels of 5–8 mol% to achieve high-temperature phase stability. Historically, this system has been valued for its robust cubic structure and tolerance to aggressive thermal environments.
At temperatures above 1600 °C, calcia stabilized zirconia maintains dimensional stability and exhibits fracture toughness values around 4–6 MPa·m¹ᐟ². Additionally, chemical interaction with molten metals remains limited when impurity content is controlled below 0.2 wt%. Nevertheless, long-term aging can induce secondary phase precipitation at grain boundaries, particularly after 200+ hours near 1200 °C. Consequently, careful composition control is necessary to sustain performance across repeated cycles.
Accordingly, calcia stabilized zirconia serves specialized applications where extreme temperature exposure outweighs cycling frequency considerations.
Tradeoffs among ionic conductivity strength and aging
Stabilizer choice introduces inherent tradeoffs between ionic conductivity, mechanical robustness, and aging resistance. Yttria stabilized zirconia exhibits higher oxygen vacancy mobility, enabling stress relaxation but slightly increasing susceptibility to low-temperature aging below 400 °C. Conversely, magnesia stabilized systems reduce vacancy mobility, enhancing creep resistance while narrowing cooling tolerance.
Comparative studies indicate that ionic conductivity values range from 0.01–0.1 S·cm⁻¹ at 1000 °C, depending on stabilizer content. Higher conductivity promotes stress accommodation during thermal gradients, yet excessive vacancy concentration accelerates grain boundary diffusion. Therefore, stabilizer systems must balance conductivity-driven relaxation against long-term microstructural stability.
Ultimately, performance classes should be selected based on dominant thermal profiles rather than nominal maximum temperature ratings.
Stabilizer system performance comparison for melting and casting
| Stabilizer system | Typical stabilizer content (mol%) | Stable phase range (°C) | Thermal cycling tolerance (cycles) | Key performance emphasis |
|---|---|---|---|---|
| Yttria stabilized | 3–8 Y₂O₃ | 25–1700 | > 100 | Broad cycling stability |
| Magnesia stabilized | 8–10 MgO | 600–1800 | 60–80 | Long soak resistance |
| Calcia stabilized | 5–8 CaO | 800–2000 | 40–60 | Extreme temperature stability |

Thermomechanical Properties That Protect Stability
Thermomechanical properties translate zirconia’s crystal chemistry into measurable resistance against melting and casting stresses. Moreover, these properties determine how internal stresses form, redistribute, or relax under sustained heat and transient gradients. Consequently, quantifiable parameters provide the most direct evidence linking material science to observed stability.
Thermal expansion and stress accommodation
Thermal expansion governs how zirconia responds to temperature gradients across the crucible wall. Stabilized zirconia exhibits a linear thermal expansion coefficient typically within 9.5–11.0 × 10⁻⁶ K⁻¹ between 25 °C and 1500 °C, which remains relatively uniform across stabilized compositions.
This moderate expansion rate limits mismatch stresses when temperature gradients reach 100–200 °C during melt holding or pouring. Furthermore, uniform expansion reduces differential strain accumulation between inner hot faces and cooler outer surfaces. As a result, internal stresses distribute gradually rather than concentrating at geometric discontinuities. Consequently, zirconia crucibles maintain dimensional coherence even when thermal profiles deviate from ideal symmetry.
Thus, controlled thermal expansion acts as the first mechanical buffer against gradient-induced cracking.
Thermal conductivity and gradient control
Thermal conductivity influences how rapidly heat redistributes through the crucible body. Dense stabilized zirconia typically shows conductivity values of 2.0–2.5 W·m⁻¹·K⁻¹ at 1000 °C, decreasing slightly at higher temperatures due to phonon scattering.
Lower conductivity slows heat flow, thereby dampening abrupt temperature changes during pouring. However, excessively low conductivity could intensify localized gradients. Zirconia occupies a balanced regime where heat transfer is sufficient to smooth long-term gradients while still moderating short-term thermal shocks. Consequently, radial temperature differences remain within manageable limits during both holding and transient cooling phases.
Therefore, thermal conductivity functions as a regulator rather than a simple insulator in melting and casting stability.
Elastic modulus strength and fracture toughness
Elastic modulus defines stiffness, while fracture toughness governs resistance to crack propagation. Stabilized zirconia exhibits elastic modulus values around 200–210 GPa at room temperature, decreasing to approximately 150–170 GPa at 1400 °C.
Simultaneously, fracture toughness remains comparatively high, typically 6–10 MPa·m¹ᐟ², depending on stabilizer system and grain size. This combination allows zirconia to sustain elastic deformation under stress while resisting crack extension when local tensile stresses arise. Moreover, toughness retention at elevated temperature prevents microcracks from coalescing during repeated thermal cycles. Consequently, the material tolerates both stiffness-driven load bearing and energy dissipation under transient stress.
Hence, the modulus–toughness balance directly underpins zirconia’s stability during coupled melting and casting operations.
Creep resistance during extended melt holding
Creep resistance determines how zirconia responds to sustained stress at high temperature. During prolonged melt holding at 1450–1600 °C, crucibles experience constant gravitational and thermal stresses that may induce time-dependent deformation.
Stabilized zirconia demonstrates steady-state creep rates typically below 1 × 10⁻⁷ s⁻¹ under compressive stresses of 5–10 MPa at 1500 °C. Furthermore, controlled creep allows gradual stress relaxation without introducing sudden strain localization. This behavior contrasts with brittle ceramics, where limited creep capacity can lead to abrupt failure once elastic limits are exceeded. Consequently, zirconia preserves geometry over extended holding periods without sagging or distortion.
Accordingly, creep resistance completes the thermomechanical framework that protects stability during long-duration melting.
Thermomechanical property ranges relevant to melting and casting
| Property | Typical value range | Reference temperature (°C) | Stability contribution |
|---|---|---|---|
| Thermal expansion coefficient (×10⁻⁶ K⁻¹) | 9.5–11.0 | 25–1500 | Gradient stress accommodation |
| Thermal conductivity (W·m⁻¹·K⁻¹) | 2.0–2.5 | 1000 | Gradient moderation |
| Elastic modulus (GPa) | 150–210 | 25–1400 | Load-bearing stiffness |
| Fracture toughness (MPa·m¹ᐟ²) | 6–10 | 1200–1400 | Crack resistance |
| Creep rate (s⁻¹) | ≤ 1 × 10⁻⁷ | 1500 | Long-term dimensional stability |

Microstructure Features That Sustain Service Life
Microstructure determines how bulk properties manifest during service. Moreover, crucibles sharing identical stabilizer chemistry can display markedly different lifetimes because density, grain architecture, and defect populations govern stress distribution. Consequently, service stability depends as much on microstructural control as on nominal material composition.
Density and closed porosity effects
Bulk density controls the availability of pathways for heat, stress, and chemical species. High-performance zirconia crucibles typically achieve relative densities above 98.5–99.5 %, limiting interconnected porosity that would otherwise concentrate stress.
Closed porosity below 1.5 vol% reduces local compliance mismatches during thermal expansion. Furthermore, pores larger than 20–30 μm act as stress amplifiers under gradients exceeding 100 °C, whereas finer closed pores distribute strain more uniformly. As a result, dense microstructures suppress crack nucleation during repeated heating cycles and maintain stiffness during prolonged melt holding.
Therefore, density acts as a foundational requirement for sustaining long service life under melting and casting conditions.
Grain size distribution and crack growth rate
Grain size directly influences fracture behavior and phase stability. Stabilized zirconia used for melting and casting commonly targets average grain sizes between 0.3–0.8 μm, a range that preserves transformation toughening while avoiding excessive grain boundary sliding.
Experimental crack propagation studies show that reducing grain size from 1.2 μm to 0.6 μm can decrease crack growth rates by nearly 40–60 % under cyclic thermal loading at 1400 °C. Moreover, narrow grain size distributions minimize local stiffness variation, preventing preferential crack paths. Consequently, uniform grains delay the transition from microcrack initiation to unstable fracture.
Thus, grain size control functions as a microstructural brake on crack evolution during aggressive thermal cycling.
Impurity phases and high temperature embrittlement
Minor impurity phases can significantly influence high-temperature behavior. Silica, alkali oxides, or residual glassy phases above 0.1–0.2 wt% often segregate at grain boundaries, lowering softening temperature and accelerating embrittlement.
At temperatures exceeding 1300 °C, such phases can soften or volatilize, weakening grain boundary cohesion and facilitating intergranular cracking. In contrast, zirconia crucibles engineered with impurity levels below 0.05 wt% maintain clean grain boundaries and stable mechanical response even after 100+ hours of exposure. Consequently, impurity suppression directly correlates with extended service intervals.
Accordingly, microstructural purity remains a decisive factor in sustaining stability beyond initial qualification tests.
Microstructural parameters influencing service stability
| Microstructural feature | Quantitative range | Reference condition | Stability contribution |
|---|---|---|---|
| Relative density (%) | 98.5–99.5 | As-sintered | Suppresses stress concentrators |
| Closed porosity (vol%) | ≤ 1.5 | As-sintered | Limits crack initiation |
| Average grain size (μm) | 0.3–0.8 | Post-sintering | Controls crack growth |
| Impurity content (wt%) | ≤ 0.05–0.1 | Bulk composition | Prevents embrittlement |

Stability Evidence in Precious Metal Melting
Precious metal melting imposes uniquely stringent stability criteria. Moreover, melt cleanliness and interfacial neutrality dominate performance evaluation because even trace reactions manifest as casting defects. Consequently, zirconia stability in this context is validated by its ability to preserve chemical purity and structural integrity across repeated noble metal heats.
Platinum group metal melt behavior and crucible interaction
Platinum group metals operate at melting temperatures typically between 1550–1750 °C, where crucible–melt interaction becomes a primary concern. Dense stabilized zirconia demonstrates minimal wetting against platinum and palladium melts, with observed contact angles exceeding 120° at operating temperatures.
Under sustained holding periods of 30–90 minutes, interfacial reaction layers remain limited to thicknesses below 2 μm, indicating diffusion-controlled rather than reaction-driven interaction. Furthermore, zirconia maintains phase stability without reduction or oxygen depletion despite prolonged exposure to molten platinum. As a result, crucible walls retain mechanical coherence while preventing melt adhesion that would otherwise induce localized stress during pouring.
Therefore, weak wetting and slow interfacial kinetics confirm zirconia’s suitability for platinum group metal processing where interface neutrality is essential.
Gold silver casting cleanliness and defect suppression
Gold and silver casting emphasizes surface finish and inclusion control rather than extreme temperature endurance. Typical operating temperatures range from 1000–1150 °C, yet contamination sensitivity remains high because defects directly translate into visible surface flaws.
Analytical melt sampling after repeated heats shows zirconia-derived contamination levels below 5 ppm, provided crucible impurity content remains under 0.05 wt%. Moreover, the absence of silica-rich phases prevents glassy film formation that could otherwise dissolve into noble metal melts. Consequently, castings exhibit reduced pinholes and surface inclusions compared with alumina-based alternatives under equivalent thermal conditions.
Thus, zirconia crucibles support defect suppression by maintaining chemical isolation between ceramic and melt throughout multiple casting cycles.
Practical stability indicators in repeated precious metal heats
Operational stability in precious metal environments is often assessed through indirect indicators rather than destructive testing. Dimensional measurements after 20–50 cycles commonly show height and diameter deviations below 0.1 %, reflecting limited creep and uniform thermal expansion.
Additionally, surface inspections reveal minimal roughening when grain size remains below 0.8 μm and density exceeds 99 %. Acoustic response during handling also remains consistent, indicating preserved elastic integrity. Consequently, these repeatable indicators collectively demonstrate that zirconia maintains functional stability without progressive degradation under noble metal casting regimes.
Accordingly, performance evidence in precious metal melting confirms that zirconia’s physical and chemical attributes translate into observable, repeatable stability metrics.
Stability metrics observed in precious metal melting
| Evaluation parameter | Typical observed range | Measurement context | Stability significance |
|---|---|---|---|
| Contact angle (°) | > 120 | 1550–1700 °C melt | Weak wetting |
| Interfacial layer thickness (μm) | < 2 | 60 min holding | Limited reaction |
| Melt contamination (ppm) | < 5 | Multi-cycle casting | Purity preservation |
| Dimensional deviation (%) | < 0.1 | 20–50 cycles | Structural stability |
| Surface roughness change (μm) | < 1 | Post-service inspection | Minimal degradation |

Stability Evidence in Superalloy Melting and Casting
Superalloy melting and casting impose more severe thermal and mechanical demands than noble metal systems. Moreover, stability is governed by sustained high-temperature exposure combined with aggressive thermal cycling. Consequently, zirconia performance in this domain is validated by its ability to tolerate intense heat loads without phase destabilization or structural distortion.
Nickel base alloy thermal load characteristics
Nickel base superalloys typically melt and cast within 1500–1650 °C, often requiring extended soak periods to ensure compositional homogeneity. During these holds, crucibles experience continuous thermal stress under significant melt mass and electromagnetic stirring.
Dense stabilized zirconia exhibits dimensional stability during soak durations of 60–180 minutes, with measured creep-induced deformation remaining below 0.2 % after 40–60 cycles. Furthermore, radial temperature gradients of 80–160 °C do not induce observable phase transformation or cracking when grain size is controlled below 0.8 μm. As a result, zirconia maintains load-bearing integrity while accommodating thermal gradients inherent to nickel alloy processing.
Therefore, zirconia’s resistance to creep and phase instability supports its sustained use in nickel base superalloy melting environments.
Cobalt base alloy reactivity tendencies and control
Cobalt base alloys present additional chemical challenges due to higher oxygen affinity and active alloying elements. Operating temperatures commonly range from 1400–1600 °C, with frequent adjustments during composition tuning.
Under these conditions, stabilized zirconia demonstrates controlled interfacial behavior, with reaction layer growth rates typically below 2–3 μm/hour. Moreover, oxide stability prevents dissolution or chemical reduction even when oxygen potential fluctuates during alloy additions. Consequently, crucible walls retain mechanical strength while limiting chemical exchange that could compromise melt quality.
Thus, zirconia provides a chemically inert containment medium that remains stable despite the reactive tendencies of cobalt-based superalloys.
Stability indicators under aggressive thermal cycling
Superalloy casting often involves rapid transitions between heating, holding, and pouring stages. Thermal cycling tests simulating 25–75 cycles between room temperature and 1600 °C reveal that stabilized zirconia maintains phase integrity above 95 % tetragonal/cubic fraction throughout the cycling regime.
Additionally, fracture inspections show that crack initiation rates remain low when thermal ramp variations are kept within ±20 % of programmed profiles. Elastic modulus retention above 85 % of initial values further confirms structural resilience. Consequently, zirconia demonstrates consistent stability even under aggressive and repetitive thermal loading.
Accordingly, stability evidence from superalloy melting and casting confirms that zirconia’s material attributes effectively translate into reliable performance under extreme metallurgical conditions.
Stability performance metrics in superalloy applications
| Evaluation parameter | Typical observed range | Operating context | Stability implication |
|---|---|---|---|
| Operating temperature (°C) | 1500–1650 | Melt holding | High thermal tolerance |
| Creep deformation (%) | < 0.2 | 40–60 cycles | Dimensional retention |
| Reaction layer growth (μm/h) | < 3 | Alloy holding | Chemical inertness |
| Phase retention (%) | > 95 | Thermal cycling | Structural coherence |
| Modulus retention (%) | > 85 | Post-cycling | Mechanical stability |

Geometry Effects on Stable Heat Flow
Crucible geometry governs how heat moves and concentrates during melting and casting. Moreover, geometric proportions shape temperature fields and stress paths without altering material chemistry. Consequently, stability emerges when geometry supports uniform heat flow rather than amplifying gradients at critical regions.
Wall thickness and thermal gradient shaping
Wall thickness directly controls radial temperature gradients between the hot inner surface and the cooler exterior. In zirconia crucibles, typical wall thickness ranges between 6–15 mm for laboratory and industrial casting formats.
Thermal modeling indicates that increasing wall thickness from 6 mm to 12 mm can raise radial gradients by 30–60 % during steady melt holding at 1500 °C, because heat dissipation slows across the ceramic body. However, thinner walls below 5 mm intensify thermal shock sensitivity during pouring transitions, where cooling rates may exceed 10 °C/s. As a result, optimized thickness balances gradient magnitude against transient shock tolerance rather than minimizing thickness alone.
Therefore, wall thickness acts as a geometric moderator that stabilizes heat flow when aligned with furnace power density and melt mass.
Height to diameter ratio and convection patterns
The height-to-diameter ratio influences internal convection within the molten metal and external heat loss along the crucible wall. Ratios between 1.0–1.6 are commonly observed in stable zirconia crucible designs for melting and casting.
When the ratio exceeds 1.8, vertical gradients intensify, leading to temperature differences above 150 °C between upper and lower wall sections during melt holding. Conversely, shallow geometries with ratios below 0.8 promote excessive surface heat loss during pouring, increasing rim stress. Computational simulations show that balanced ratios maintain convection-driven heat redistribution, reducing localized hot spots and cold zones.
Thus, geometric proportion shapes the thermal environment in which zirconia properties operate, reinforcing overall stability.
Lip and rim robustness during pouring transitions
The rim and lip region experiences the most severe thermal and mechanical disturbances during pouring. Typical lip radii between 3–6 mm significantly reduce stress concentration compared with sharp edges below 1 mm.
Experimental strain measurements indicate that rounded rims lower peak tensile stress by 25–40 % during rapid pouring-induced cooling. Furthermore, thicker lip sections maintain thermal mass, delaying abrupt temperature drops that trigger microcracking. Consequently, geometric reinforcement at the rim preserves structural continuity through repeated pour cycles.
Accordingly, rim geometry becomes a decisive factor in stabilizing heat flow and stress distribution during casting transitions.
Geometric parameters influencing thermal stability
| Geometric parameter | Typical range | Thermal effect | Stability contribution |
|---|---|---|---|
| Wall thickness (mm) | 6–15 | Radial gradient control | Shock–gradient balance |
| Height/diameter ratio | 1.0–1.6 | Convection moderation | Uniform heat flow |
| Rim radius (mm) | 3–6 | Stress concentration reduction | Pouring durability |
| Lip thickness (mm) | ≥ wall thickness | Thermal mass retention | Crack suppression |

Surface Integrity and Chemical Inertness
Surface integrity determines whether bulk stability assumptions remain valid in service. Moreover, the crucible surface is the first interface exposed to molten metal, thermal gradients, and oxygen activity. Consequently, microscopic surface conditions can either preserve chemical inertness or undermine otherwise robust material properties.
Surface finish and crack initiation probability
Surface finish directly influences crack initiation under thermal stress. Zirconia crucibles finished to average roughness values below Ra 0.8–1.2 μm exhibit substantially lower microcrack density after repeated thermal cycling compared with surfaces exceeding Ra 2.5 μm.
High-magnification inspections after 30–60 melting cycles reveal that machining grooves deeper than 10–15 μm act as preferential crack origins during pouring transitions. Furthermore, smoother surfaces distribute thermal strain more evenly, reducing localized tensile peaks when cooling rates exceed 8–12 °C/s at the rim. As a result, surface refinement delays crack nucleation even when bulk stresses approach critical thresholds.
Therefore, surface finish operates as a probabilistic control factor that governs whether internal stresses convert into visible damage.
Surface chemistry evolution at peak temperature
At elevated temperatures, zirconia surfaces undergo subtle chemical reorganization. Oxygen vacancy redistribution becomes significant above 1300 °C, influencing surface energy and interaction with molten metals.
Spectroscopic analyses indicate that stabilized zirconia maintains stoichiometric surface composition within ±0.5 at% oxygen deviation after prolonged exposure at 1500–1600 °C. Moreover, the absence of reducible secondary oxides prevents the formation of chemically active patches that could catalyze interfacial reactions. Consequently, surface chemistry remains inert despite extended thermal exposure, preserving boundary stability during melt holding.
Thus, chemical steadiness at the surface reinforces zirconia’s role as a passive containment medium rather than a reactive participant.
Glaze free surfaces and wetting sensitivity
Glassy or glazed surface layers introduce compositional heterogeneity that alters wetting behavior. Zirconia crucibles with residual glassy phases above 0.1 wt% show reduced contact angles by 15–25° when exposed to noble metal melts.
Such wetting enhancement promotes localized adhesion and increases stress during pouring as molten metal detaches from the wall. In contrast, glaze-free zirconia surfaces maintain high contact angles above 110°, minimizing capillary penetration and mechanical coupling with the melt. Consequently, surface chemical uniformity directly supports inert wetting behavior under casting conditions.
Accordingly, glaze-free surfaces preserve the validity of zirconia’s chemical stability assumptions during repeated melting and casting cycles.
Surface-related parameters influencing stability
| Surface parameter | Quantitative range | Reference condition | Stability contribution |
|---|---|---|---|
| Surface roughness Ra (μm) | 0.8–1.2 | As-finished | Reduces crack initiation |
| Machining groove depth (μm) | < 10–15 | Rim and wall | Limits stress concentration |
| Oxygen deviation (at%) | ± 0.5 | 1500–1600 °C exposure | Chemical inertness |
| Glassy phase content (wt%) | ≤ 0.1 | Bulk surface | Maintains weak wetting |

Practical Methods to Verify Stability in Use
Stability claims require verification under real operating conditions. Moreover, practical validation relies on measurable indicators rather than abstract material specifications. Consequently, simple monitoring methods allow engineers to confirm whether zirconia maintains structural and chemical coherence during melting and casting.
Mass change and dimensional drift monitoring
Mass stability provides an indirect measure of chemical interaction and material loss. In controlled melting and casting operations, stabilized zirconia crucibles typically exhibit mass changes below 0.02–0.05 % after 20–40 cycles at temperatures exceeding 1500 °C.
Dimensional drift offers complementary insight into creep and phase stability. Measurements of height and diameter after repeated heats generally remain within ±0.1 % of original dimensions when creep rates stay below 1 × 10⁻⁷ s⁻¹. Furthermore, consistent dimensional retention across cycles indicates balanced thermal expansion and stress relaxation. As a result, combined mass and dimensional tracking establishes a baseline for long-term stability assessment.
Therefore, routine measurement before and after service intervals confirms whether zirconia performance remains within expected stability bounds.
Visual indicators and microcrack screening logic
Visual inspection serves as an efficient screening method when guided by quantitative criteria. Under stable conditions, surface coloration remains uniform, and roughness increase stays below 1 μm Ra after extended use.
Magnified inspection at 10×–20× reveals whether microcrack density exceeds acceptable thresholds. Field observations indicate that crack spacing greater than 5–10 mm without interconnection corresponds to stable service behavior, whereas dense crack networks signal stress accumulation beyond material accommodation. Moreover, consistent acoustic response during handling reflects preserved elastic modulus above 85 % of baseline values. Consequently, visual and tactile indicators together provide rapid confirmation of structural integrity.
Thus, systematic visual screening translates qualitative observation into actionable stability verification.
Simple thermal cycling validation protocol
Thermal cycling tests replicate service conditions in an accelerated manner. A practical validation protocol involves cycling between ambient temperature and 1400–1600 °C for 10–20 cycles, using ramp rates consistent with operational practice.
Successful validation is indicated by phase retention above 95 %, absence of new surface cracks, and dimensional change below 0.05 % after completion. Additionally, thermal imaging during cycling should show gradient symmetry without localized hot spots exceeding 150 °C differential. Consequently, such protocols allow stability confirmation without extensive laboratory instrumentation.
Accordingly, simple cycling validation bridges theoretical material stability with observed performance in melting and casting environments.
Practical verification metrics for stability confirmation
| Verification method | Typical acceptance range | Observation interval | Stability confirmation |
|---|---|---|---|
| Mass change (%) | ≤ 0.05 | 20–40 cycles | Chemical inertness |
| Dimensional drift (%) | ≤ ±0.1 | Multi-cycle | Creep control |
| Surface roughness increase (μm) | ≤ 1 | Post-service | Structural coherence |
| Phase retention (%) | ≥ 95 | Post-cycling | Phase stability |
| Gradient symmetry (°C) | ≤ 150 | During cycling | Thermal balance |
Common Questions Engineers Ask During Use
What makes zirconia crucible stable at casting temperatures
Zirconia stability at casting temperatures originates from phase-stabilized crystal structures that suppress volumetric transformation above 1400 °C. Moreover, moderate thermal expansion coefficients and high fracture toughness distribute stresses induced by gradients of 100–200 °C. Consequently, structural coherence is preserved during melt holding and pouring transitions.
Does stabilized zirconia behave differently in repeated melts
Stabilized zirconia demonstrates consistent behavior across repeated melts because tetragonal or cubic phases remain above 95 % after 50–100 cycles. Furthermore, controlled creep rates below 1 × 10⁻⁷ s⁻¹ allow gradual stress relaxation without geometric distortion. As a result, dimensional drift typically remains within ±0.1 % over extended service.
Why does some zirconia show surface roughening after heats
Surface roughening arises when grain boundary phases soften or volatilize above 1300 °C. In particular, impurity contents exceeding 0.1 wt% can promote localized grain pullout and roughness increases above 1 μm Ra. Therefore, microstructural purity and dense sintering directly influence surface stability during prolonged heating.
Which stabilizer system tolerates thermal cycling better in casting
Yttria stabilized zirconia generally tolerates frequent thermal cycling most effectively, sustaining 100+ cycles between ambient temperature and 1500 °C with minimal phase degradation. Conversely, magnesia stabilized systems favor long soak stability but require controlled cooling through intermediate temperature ranges. Thus, cycling tolerance depends on stabilizer chemistry and thermal profile alignment.
Can zirconia preserve melt purity during precious metal casting
Zirconia preserves melt purity by exhibiting low solubility and weak wetting toward noble metal melts. Analytical sampling commonly shows contamination levels below 5–10 ppm after repeated casting cycles. Consequently, compositional fidelity and surface finish quality are maintained throughout precious metal processing.
Conclusion
Overall, zirconia crucible stability in melting and casting arises from phase control, thermomechanical balance, chemical inertness, and microstructural integrity acting together across extreme thermal environments.
References:
-
In physics and engineering, a transient describes a temporary, non-steady-state response of a system as it transitions between equilibrium conditions, often involving rapid changes in temperature, stress, or energy distribution. ↩
-
Oxygen potential refers to the thermodynamic tendency of a system to gain or lose oxygen, governing oxide stability, interfacial reactions, and chemical equilibrium in high-temperature metallurgical and ceramic environments. ↩