Ceramic Laboratory Crucible for High-Temperature Analytical Testing and Thermal Treatment
Metal melting crucibles often fail under thermal cycling from cracking, hot-face erosion, and inclusion pickup—causing downtime, metal loss, and unstable melt quality.
ADCERAX engineers ceramic metal melting crucibles in alumina, silicon carbide, and zirconia to match your alloy chemistry and furnace heat profile, controlling ∆T-driven stress and crucible–melt interaction for cleaner, repeatable melts from 700–1800°C.
We engineer ceramic crucibles that last 2x longer and eliminate contamination.
Where Metal Crucibles Fail?
Stainless steel crucibles are often used for high-temperature ashing, LOI, and residue tests, but in muffle furnaces they create repeatability problems.
🔹Scale + discoloration under oxygen and thermal cycling
🔹Oxide flakes or adheres to residue, causing mass gain and gravimetric error
🔹Pitting / local thinning with fluxes, alkali salts, or corrosive decomposition products
🔹Frequent replacement to manage failure, which adds run-to-run variability and cross-contamination risk between “old vs new” crucibles

Causes of Metal Crucible Failure
Under sustained high-temperature use, metal crucibles degrade through well-defined physical and chemical mechanisms. Oxidation, creep, and surface reactions alter mass, geometry, and surface condition, directly impacting repeatability and contamination control in laboratory testing.
Metal oxidation during high-temperature exposure
- Oxide scale grows and can spall under cycling, shifting crucible mass and shedding particles into the sample zone.
Thermal creep and softening near service limits
- Long dwell cycles can cause rim ovalization or base warp, altering contact conditions and heating repeatability.
Chemical attack by alkali/halide vapors and aggressive residues
- Pitting and surface roughening increase residue adhesion and accelerate degradation.
Metal–sample interaction
- Certain oxides and salts can alloy, wet, or chemically react with metal, increasing contamination risk and residue retention.
Cleaning-driven surface damage
- Abrasive cleaning and repeated chemical cleaning can roughen surfaces, increasing carryover and worsening run-to-run variance.
Why Ceramic Laboratory Crucible Solves This Failure?
Ceramic crucibles address contamination, thermal stability, and measurement repeatability requirements in high-temperature laboratory analysis. Material selection directly affects tare stability, sample interaction, and result consistency across thermal cycles—particularly critical under oxidizing atmospheres where conventional materials fail.
Ceramic Crucible Advantages for Laboratory
Ceramics avoid oxidation-driven mass change, helping maintain consistent tare values across repeated laboratory heating cycles.
Ceramic surfaces are less prone to wetting or chemical reaction with many residues, reducing carryover and contamination risk.
Ceramics retain stiffness and geometry at typical laboratory heat-treatment temperatures, supporting repeatable positioning and handling.
Different Laboratory Ceramic Crucible Material Options

Stable mass and geometry at high temperature; good general chemical stability for routine lab furnace use.

Lower interaction with some residues; improved resistance for more aggressive chemistries when grade and cycling are controlled.

Better thermal-shock tolerance for fast ramps and frequent cycling; verify atmosphere/residue compatibility for the method.
How to Choose a Ceramic Crucible for Laboratory?
A durable solution requires balancing thermal loading, chemistry, and geometry rather than optimizing one property in isolation. The framework below is designed to help engineers specify a ceramic metal melting crucible that remains stable across real operating variability.
Key Selection Parameters
Engineers must evaluate these interdependent factors:
Operating temperature profile: peak temperature, dwell time, ramp rate, and cycle frequency; faster ramps increase thermal shock risk, especially with thicker walls.
Atmosphere: air/oxygen vs inert; volatile alkali/halide species can increase chemical attack and surface glazing risk.
Test /process purpose: heat treatment, calcination, sintering trials, powder pre-conditioning, or residue handling; this drives lid need and cleaning requirements.
Contamination sensitivity: whether trace-level interference or carryover must be controlled; defines purity level and cleaning protocol rigor.
Geometry constraints: capacity, wall/base thickness, rim profile, lid design, and whether a covered configuration is required.
Laboratory Ceramic Crucible Material Comparison
The following materials are commonly specified, each with distinct performance profiles:
| Material | Strengths | Limitations | Best-fit Conditions | Notes |
|---|---|---|---|---|
| High-purity Alumina | Stable mass in oxidizing heat; good general chemical stability; maintains geometry at high temperature | Can be attacked by certain alkali fluxes/vapors; thermal shock resistance depends on thickness and cycling | General laboratory heat treatment and calcination in air; broad residue types; steady heating profiles | Often paired with lids to reduce spatter and airborne pickup |
| Zirconia | Strong chemical resistance for selected residues; good high-temperature stability with appropriate grade | Some grades can be sensitive to rapid thermal gradients; higher density increases thermal mass | More aggressive residues where alumina shows glazing/attack; controlled cycling | Validate compatibility with specific salts/fluxes and method chemistry |
| Silicon Carbide | Excellent tolerance for rapid cycling and thermal shock; high thermal conductivity | Oxidation behavior and surface changes depend on temperature and atmosphere; compatibility varies with chemistry | Fast ramp rates; frequent cycling; robust handling environments | Consider methods where surface condition must remain consistent across runs |
Which Ceramic Crucible Configuration Fits for Laboratory?
Engineers specify ceramic crucibles in forms determined by furnace type, capacity requirements, and material handling systems. The following configurations represent standard solutions adapted to common industrial melting scenarios.
High Form Ceramic Crucible for Lab
Deep charge holding; less spill risk. For furnace heat treatment and long soaks.
Low Form Ceramic Crucible for Lab
Faster heating; easier loading/cleaning. For shallow samples and quick cycles.
Semicircular Ceramic Crucible for Lab
Stable support for long/narrow samples. For tube-furnace zones and directional heating.
Rectangular Ceramic Crucible for Lab
Maximizes usable volume and stacking. Use for batch heating powders and solids.
Ceramic Crucible with Spout for Lab
Controlled pouring with less residue loss. Use when molten material must transfer cleanly.
Point Bottom Ceramic Crucibles for lab
Improved drainage with less residue retention. Use for small samples and recovery.
Calcination Crucible
Powder heat treatment, phase transformation, and thermal conditioning.
DSC Ceramic Crucible
Differential scanning calorimetry to measure heat flow and transitions.
Where Laboratory Ceramic Crucibles Are Used?
These ceramic melting crucibles are used where thermal cycling, contamination risk, and downtime costs exceed conventional refractory limits. The scenarios below reflect typical operating contexts for advanced ceramics.
Loss-on-ignition testin
Measurement of mass loss during heating to 950–1050 °C to quantify carbonate decomposition, bound water, or organic fraction in minerals, soils, and industrial materials.
Thermal analysis preparation
Sample pre-treatment for TGA and DTA requiring crucibles with known thermal mass, minimal reactivity, and dimensional consistency.
Materials synthesis and powder calcination
Laboratory-scale calcination of ceramic precursors, catalyst activation, and thermal decomposition studies requiring contamination-free heating and precise temperature control.
Fusion flux methods
High-temperature reaction of sample with lithium borate, sodium peroxide, or other fluxing agents at 900–1200 °C to render refractory oxides soluble for subsequent analysis.
Battery materials processing
High-temperature treatment of lithium transition metal oxide precursors for cathode materials at 700–900 °C, requiring contamination-free processing in oxidizing atmospheres.
Spectroscopic sample preparation
Pre-combustion or calcination of biological, environmental, and geological samples before ICP-MS, ICP-OES, or XRF to remove organics and standardize sample form.
Laboratory Ceramic Crucible Failure Modes & Mitigation Guide
When a Ceramic Laboratory Crucible shows cracking, sticking, or mass drift, the cause is usually thermal gradient, chemistry interaction, or handling damage. The table below maps each symptom to a corrective action that can be implemented without changing the full method.
| Symptom | Likely Cause | Design / Material Adjustment | Notes |
|---|---|---|---|
| Tare mass drifts between runs | Deposits/glaze formation, inconsistent cleaning, airborne pickup | Standardize cleaning/burn-off; consider zirconia for aggressive residues; use lids where appropriate | Track tare history per crucible to identify outliers |
| Cracking after thermal cycling | Ramp rates too aggressive for geometry; local gradients from uneven seating | Reduce ramp rate; adjust wall/base thickness; consider SiC for fast cycles | Ensure flat seating and avoid point contacts in fixtures |
| Residue adheres strongly / difficult release | Wetting or reaction between residue and ceramic surface | Change material (alumina → zirconia); reduce peak temperature/dwell; consider containment approach | Validate with representative residues before scaling |
| Rim chipping during handling | Tong impact, lid misalignment, stacking without separators | Specify stronger rim geometry; improve handling clearance; use separators | Rim damage can propagate during subsequent cycles |
| Surface glazing builds up | Alkali vapors/salts reacting with surface | Use covered geometry; change material grade; add secondary containment | Glazing can bias tare and change residue behavior |
Ceramic Metal Melting Crucible Customization
Standard crucibles rarely match real melting conditions. ADCERAX supports custom ceramic melting crucibles engineered around your metal type, thermal cycle, and furnace interface to reduce cracking, contamination, and handling risk.
Why Custom Crucibles Are Specified ?
Melting temperatures or thermal ramp rates exceed standard design limits
Crucible geometry must match furnace holders, induction coils, or clamps
Melt cleanliness or wetting behavior is critical to yield
Repeated cracking occurs at rims, corners, or mounting interfaces
Pouring, transfer, or automation requires controlled geometry
What Can Be Customized?
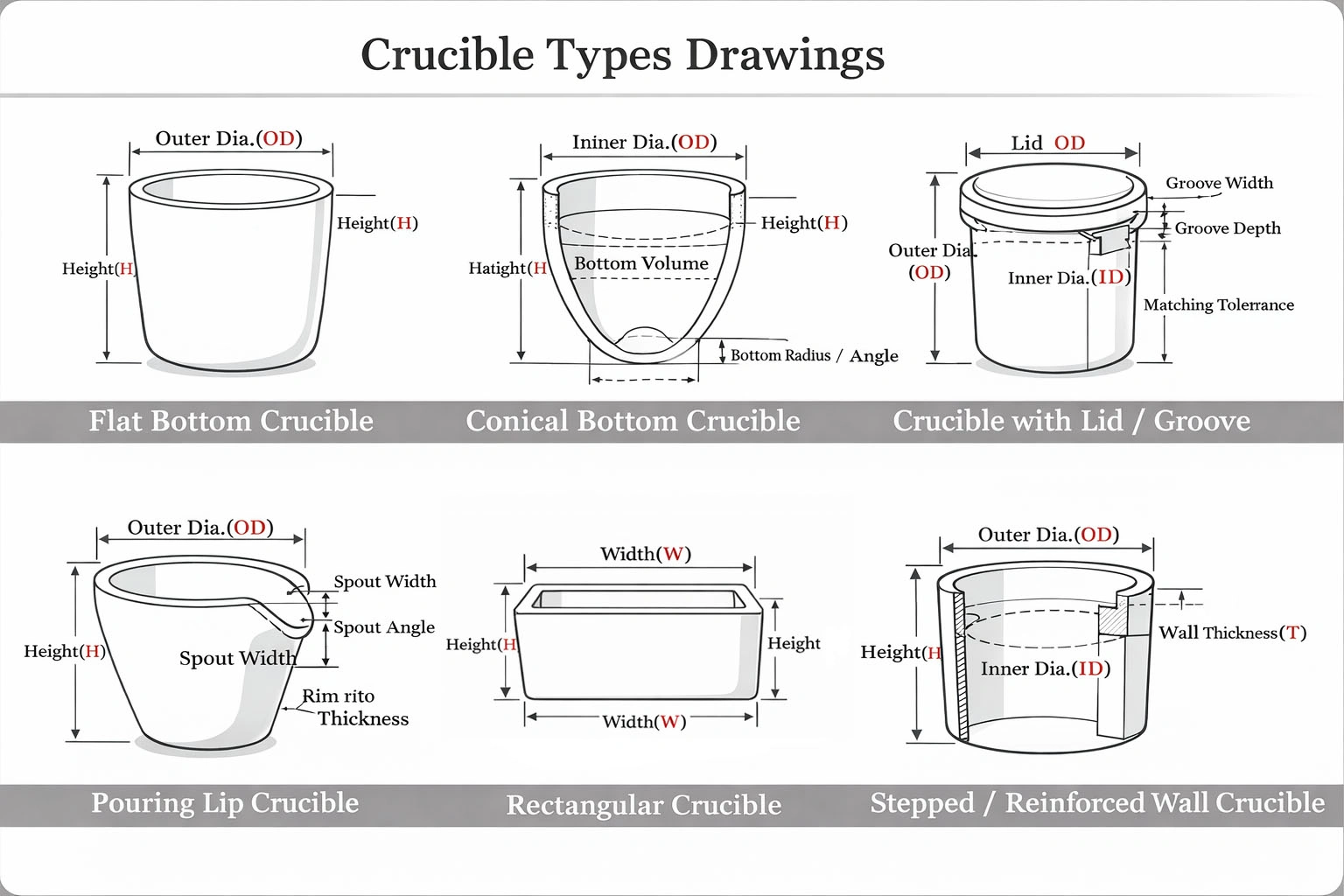
Geometry & Structure
- Inner diameter / outer diameter / height
- Wall thickness zoning (reinforced rim or base)
- Cylindrical, conical, trapezoid, rectangular, or hybrid profiles
- Flat bottom, rounded bottom, or reinforced base
- Integrated spout, lip, or pouring edge (optional)
Material & Grade
- Alumina (Al₂O₃)
- Zirconia and ZTA
- Boron nitride (BN)
- Magnesia (MgO)
- Yttria-based ceramics (Y₂O₃)
Surface & Performance Tuning
- Hot-face finish to reduce wetting or erosion
- Edge rounding to limit stress concentration
- Density and microstructure tuning for contamination-sensitive melts
Custom Ceramic Metal Melting Crucible: What to Provide?
To evaluate a custom crucible, engineers typically provide:
Metal or alloy type
Operating temperature range and cycle profile
Furnace type and mounting method
Required melt capacity
Drawing, sketch, or reference sample
Quick Acceptance Checklist for Ceramic Laboratory Crucible
| ☐ | Maximum operating temperature and hold time confirmed for the method |
| ☐ | Furnace atmosphere identified (air /oxygen/ inert) and volatility risk noted |
| ☐ | Sample chemistry screened for alkali/halide / flux interaction risk |
| ☐ | Crucible geometry selected (high-form vs low-form) based on spatter and residue handling |
| ☐ | Lid requirement defined (spatter control vs access and burn-off rate) |
| ☐ | Ramp rate and thermal cycling frequency documented for thermal shock risk review |
| ☐ | Cleaning protocol standardized (thermal burn-off vs chemical cleaning) |
| ☐ | Handling interface confirmed (tongs clearance, rim robustness, storage separators) |
| ☐ | Consistency requirement set (tare stability, batch-to-batch repeatability expectations) |